Page 43 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 43
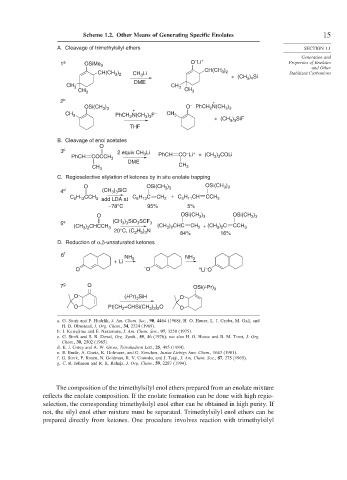
Scheme 1.2. Other Means of Generating Specific Enolates 15
A. Cleavage of trimethylsilyl ethers SECTION 1.1
Generation and
–
1 a OSiMe 3 O Li + Properties of Enolates
CH(CH ) and Other
CH(CH ) CH Li 3 2 + (CH ) Si Stabilized Carbanions
3 2
3
DME 3 4
CH 3 CH 3
CH 3 CH 3
2 b +
–
OSi(CH ) O PhCH N(CH )
3 3
2
3 3
+
CH 3 PhCH N(CH 3 ) 3 F – CH 3
2
+ (CH ) SiF
3 3
THF
B. Cleavage of enol acetates
O
3 c 2 equiv CH Li – +
PhCH COCCH 3 3 PhCH CO Li + (CH ) COLi
3 3
DME
CH 3 CH 3
C. Regioselective silylation of ketones by in situ enolate trapping
O OSi(CH ) OSi(CH )
3 3
3 3
4 d (CH ) SiCl
3 3
H C CH
C H CCH 3 add LDA at C 6 13 2 + C H CH CCH 3
5 11
6 13
–78°C 95% 5%
O OSi(CH ) OSi(CH )
3 3
3 3
3 3
3
5 e (CH ) SiO SCF 3
(CH ) CHCCH 3 (CH ) CHC CH + (CH ) C CCH 3
3 2
3 2
3 2
2
20°C, (C H ) N 84% 16%
2 5 3
D. Reduction of α,β-unsaturated ketones
6 f NH NH
+ Li 3 3
–
O – O – + Li O
7 g O OSi(i-Pr) 3
O (i-Pr) 3 SiH O
O Pt[CH =CHSi(CH ) ] O O
2
3 2 2
a. G. Stork and P. Hudrlik, J. Am. Chem. Soc., 90, 4464 (1968); H. O. House, L. J. Czuba, M. Gall, and
H. D. Olmstead, J. Org. Chem., 34, 2324 (1969).
b. I. Kuwajima and E. Nakamura, J. Am. Chem. Soc., 97, 3258 (1975).
c. G. Stork and S. R. Dowd, Org. Synth., 55, 46 (1976); see also H. O. House and B. M. Trost, J. Org.
Chem., 30, 2502 (1965).
d. E. J. Corey and A. W. Gross, Tetrahedron Lett., 25, 495 (1984).
e. E. Emde, A. Goetz, K. Hofmann, and G. Simchen, Justus Liebigs Ann. Chem., 1643 (1981).
f. G. Stork, P. Rosen, N. Goldman, R. V. Coombs, and J. Tsuji, J. Am. Chem. Soc., 87, 275 (1965).
g. C. R. Johnson and R. K. Raheja, J. Org. Chem., 59, 2287 (1994).
The composition of the trimethylsilyl enol ethers prepared from an enolate mixture
reflects the enolate composition. If the enolate formation can be done with high regio-
selection, the corresponding trimethylsilyl enol ether can be obtained in high purity. If
not, the silyl enol ether mixture must be separated. Trimethylsilyl enol ethers can be
prepared directly from ketones. One procedure involves reaction with trimethylsilyl