Page 40 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 40
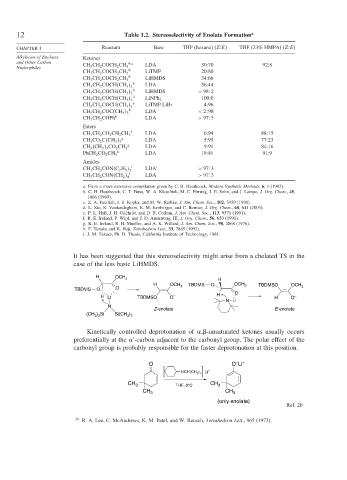
12 Table 1.2. Stereoselectivity of Enolate Formation a
CHAPTER 1 Reactant Base THF (hexane) (Z:E) THF (23% HMPA) (Z:E)
Alkylation of Enolates Ketones
and Other Carbon b c LDA 30:70 92:8
Nucleophiles CH 3 CH 2 COCH 2 CH 3
b
CH 3 CH 2 COCH 2 CH 3 LiTMP 20:80
b
CH 3 CH 2 COCH 2 CH 3 LiHMDS 34:66
b
CH 3 CH 2 COCH CH 3 2 LDA 56:44
b
CH 3 CH 2 COCH CH 3 2 LiHMDS > 98
2
d 100:0
CH 3 CH 2 COCH CH 3 2 LiNPh 2
e LiTMP.LiBr 4:96
CH 3 CH 2 COCH CH 3 2
b LDA < 2
98
CH 3 CH 2 COC CH 3 3
CH 3 CH 2 COPh b LDA > 97
3
Esters
f LDA 6:94 88:15
CH 3 CH 2 CO 2 CH 2 CH 3
g LDA 5:95 77:23
CH 3 CO 2 C CH 3 3
g LDA 9:91 84:16
CH 3 CH 2 3 CO 2 CH 3
h LDA 19:81 91:9
PhCH 2 CO 2 CH 3
Amides
CH 3 CH 2 CON C 2 H 5 i LDA i > 97
3
2
i
CH 3 CH 2 CON CH 2 4 LDA > 97
3
a. From a more extensive compilation given by C. H. Heathcock, Modern Synthetic Methods, 6, 1 (1992).
b. C. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lampe, J. Org. Chem., 45,
1066 (1980).
c. Z. A. Fataftah, I. E. Kopka, and M. W. Rathke, J. Am. Chem. Soc., 102, 3959 (1980).
d. L. Xie, K. Vanlandeghem, K. M. Isenberger, and C. Bernier, J. Org. Chem., 68, 641 (2003).
e. P. L. Hall, J. H. Gilchrist, and D. B. Collum, J. Am. Chem. Soc., 113, 9571 (1991).
f. R. E. Ireland, P. Wipf, and J. D. Armstrong, III, J. Org. Chem., 56, 650 (1991).
g. R. E. Ireland, R. H. Mueller, and A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1976).
h. F. Tanaka and K. Fuji, Tetrahedron Lett., 33, 7885 (1992).
i. J. M. Takacs, Ph. D. Thesis, California Institute of Technology, 1981.
It has been suggested that this stereoselectivity might arise from a chelated TS in the
case of the less basic LiHMDS.
H OCH
3 H
H OCH 3 TBDMS O OCH 3 TBDMSO OCH
TBDMS O O 3
H O
H Li TBDMSO O – H O –
N Li
N
Z-enolate E-enolate
(CH ) Si Si(CH )
3 3 3 3
Kinetically controlled deprotonation of , -unsaturated ketones usually occurs
preferentially at the -carbon adjacent to the carbonyl group. The polar effect of the
carbonyl group is probably responsible for the faster deprotonation at this position.
–
O O Li +
+
3 2 Li
NCH(CH )
CH 3 THF, 0°C CH 3
CH 3 CH 3
(only enolate)
Ref. 20
20
R. A. Lee, C. McAndrews, K. M. Patel, and W. Reusch, Tetrahedron Lett., 965 (1973).