Page 39 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 39
11
SECTION 1.1
N 1a N 1 Generation and
N 2
Properties of Enolates
N 2a Br 1 and Other
Li 2a Li 2 Stabilized Carbanions
O 1a
O 1
Li 1a Li 1
N 3
Si 1
Si 1a
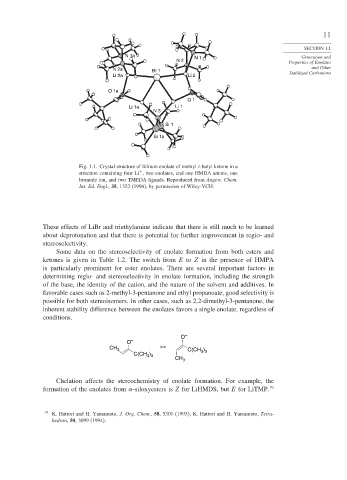
Fig. 1.1. Crystal structure of lithium enolate of methyl t-butyl ketone in a
+
structure containing four Li , two enolates, and one HMDA anions, one
bromide ion, and two TMEDA ligands. Reproduced from Angew. Chem.
Int. Ed. Engl., 35, 1322 (1996), by permission of Wiley-VCH.
These effects of LiBr and triethylamine indicate that there is still much to be learned
about deprotonation and that there is potential for further improvement in regio- and
stereoselectivity.
Some data on the stereoselectivity of enolate formation from both esters and
ketones is given in Table 1.2. The switch from E to Z in the presence of HMPA
is particularly prominent for ester enolates. There are several important factors in
determining regio- and stereoselectivity in enolate formation, including the strength
of the base, the identity of the cation, and the nature of the solvent and additives. In
favorable cases such as 2-methyl-3-pentanone and ethyl propanoate, good selectivity is
possible for both stereoisomers. In other cases, such as 2,2-dimethyl-3-pentanone, the
inherent stability difference between the enolates favors a single enolate, regardless of
conditions.
O –
O –
CH 3 >> C(CH )
C(CH ) 3 3
3 3
CH 3
Chelation affects the stereochemistry of enolate formation. For example, the
formation of the enolates from -siloxyesters is Z for LiHMDS, but E for LiTMP. 19
19
K. Hattori and H. Yamamoto, J. Org. Chem., 58, 5301 (1993); K. Hattori and H. Yamamoto, Tetra-
hedron, 50, 3099 (1994).