Page 37 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 37
at equilibrium (Entries 6 and 7). A 3-methyl group has a significant effect on the 9
regiochemistry of kinetic deprotonation but very little effect on the thermodynamic
stability of the isomeric enolates (Entry 8). SECTION 1.1
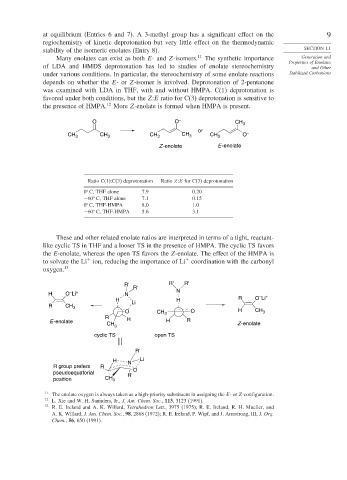
Many enolates can exist as both E- and Z-isomers. 11 The synthetic importance Generation and
Properties of Enolates
of LDA and HMDS deprotonation has led to studies of enolate stereochemistry and Other
under various conditions. In particular, the stereochemistry of some enolate reactions Stabilized Carbanions
depends on whether the E-or Z-isomer is involved. Deprotonation of 2-pentanone
was examined with LDA in THF, with and without HMPA. C(1) deprotonation is
favored under both conditions, but the Z:E ratio for C(3) deprotonation is sensitive to
the presence of HMPA. 12 More Z-enolate is formed when HMPA is present.
O O – CH 3
or
CH 3 CH 3 CH 3 CH 3 CH 3 O –
Z -enolate E -enolate
Ratio C(1):C(3) deprotonation Ratio Z:E for C(3) deprotonation
0 C, THF alone 7 9 0 20
−60 C, THF alone 7 1 0 15
0 C, THF-HMPA 8 0 1 0
−60 C, THF-HMPA 5 6 3 1
These and other related enolate ratios are interpreted in terms of a tight, reactant-
like cyclic TS in THF and a looser TS in the presence of HMPA. The cyclic TS favors
the E-enolate, whereas the open TS favors the Z-enolate. The effect of the HMPA is
+
+
to solvate the Li ion, reducing the importance of Li coordination with the carbonyl
oxygen. 13
R' R' R'
R'
–
H O Li + N N – +
H H R O Li
Li
R CH 3
O CH 3 O H CH 3
R
E- enolate H H R
CH Z- enolate
3
cyclic TS open TS
R'
H N Li
R group prefers R
pseudoequatorial R' O
position CH 3
11
The enolate oxygen is always taken as a high-priority substituent in assigning the E-or Z-configuration.
12 L. Xie and W. H. Saunders, Jr., J. Am. Chem. Soc., 113, 3123 (1991).
13
R. E. Ireland and A. K. Willard, Tetrahedron Lett., 3975 (1975); R. E. Ireland, R. H. Mueller, and
A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1972); R. E. Ireland, P. Wipf, and J. Armstrong, III, J. Org.
Chem., 56, 650 (1991).