Page 42 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 42
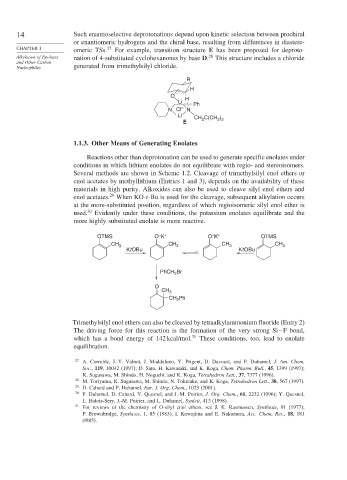
14 Such enantioselective deprotonations depend upon kinetic selection between prochiral
or enantiomeric hydrogens and the chiral base, resulting from differences in diastere-
CHAPTER 1 omeric TSs. 27 For example, transition structure E has been proposed for deproto-
28
Alkylation of Enolates nation of 4-substituted cyclohexanones by base D. This structure includes a chloride
and Other Carbon
Nucleophiles generated from trimethylsilyl chloride.
R
H
O H
Li Ph
N Cl – N
Li CH C(CH )
E 2 3 3
1.1.3. Other Means of Generating Enolates
Reactions other than deprotonation can be used to generate specific enolates under
conditions in which lithium enolates do not equilibrate with regio- and stereoisomers.
Several methods are shown in Scheme 1.2. Cleavage of trimethylsilyl enol ethers or
enol acetates by methyllithium (Entries 1 and 3), depends on the availability of these
materials in high purity. Alkoxides can also be used to cleave silyl enol ethers and
29
enol acetates. When KO-t-Bu is used for the cleavage, subsequent alkylation occurs
at the more-substituted position, regardless of which regioisomeric silyl enol ether is
used. 30 Evidently under these conditions, the potassium enolates equilibrate and the
more highly substituted enolate is more reactive.
– +
– +
OTMS O K O K OTMS
CH 3 CH 3 CH 3 CH 3
Kt OBu Kt OBu
PhCH Br
2
O
CH 3
CH Ph
2
Trimethylsilyl enol ethers can also be cleaved by tetraalkylammonium fluoride (Entry 2)
The driving force for this reaction is the formation of the very strong Si−F bond,
which has a bond energy of 142 kcal/mol. 31 These conditions, too, lead to enolate
equilibration.
27
A. Corruble, J.-Y. Valnot, J. Maddaluno, Y. Prigent, D. Davoust, and P. Duhamel, J. Am. Chem.
Soc., 119, 10042 (1997); D. Sato, H. Kawasaki, and K. Koga, Chem. Pharm. Bull., 45, 1399 (1997);
K. Sugasawa, M. Shindo, H. Noguchi, and K. Koga, Tetrahedron Lett., 37, 7377 (1996).
28 M. Toriyama, K. Sugasawa, M. Shindo, N. Tokutake, and K. Koga, Tetrahedron Lett., 38, 567 (1997).
29
D. Cahard and P. Duhamel, Eur. J. Org. Chem., 1023 (2001).
30 P. Duhamel, D. Cahard, Y. Quesnel, and J.-M. Poirier, J. Org. Chem., 61, 2232 (1996); Y. Quesnel,
L. Bidois-Sery, J.-M. Poirier, and L. Duhamel, Synlett, 413 (1998).
31
For reviews of the chemistry of O-silyl enol ethers, see J. K. Rasmussen, Synthesis, 91 (1977);
P. Brownbridge, Synthesis, 1, 85 (1983); I. Kuwajima and E. Nakamura, Acc. Chem. Res., 18, 181
(l985).