Page 44 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 44
32
16 chloride and a tertiary amine. This procedure gives the regioisomers in a ratio favoring
the thermodynamically more stable enol ether. Use of t-butyldimethylsilyl chloride
CHAPTER 1 33
with potassium hydride as the base also seems to favor the thermodynamic product.
Alkylation of Enolates Trimethylsilyl trifluoromethanesulfonate (TMS-OTf), which is more reactive, gives
and Other Carbon
Nucleophiles primarily the less-substituted trimethylsilyl enol ether. 34 Higher ratios of the less-
substituted enol ether are obtained by treating a mixture of ketone and trimethylsilyl
35
chloride with LDA at −78 C. Under these conditions the kinetically preferred enolate
is immediately trapped by reaction with trimethylsilyl chloride. Even greater prefer-
ences for the less-substituted silyl enol ether can be obtained by using the more
hindered lithium amide from t-octyl-t-butylamine (LOBA).
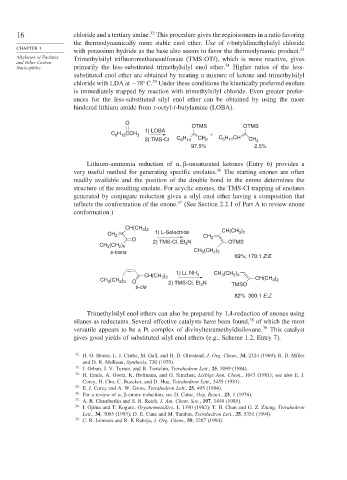
O
OTMS OTMS
1) LOBA
C H CCH 3 +
6 13
5 11
2) TMS-Cl C H CH 2 C H CH CH 3
6 13
97.5% 2.5%
Lithium-ammonia reduction of -unsaturated ketones (Entry 6) provides a
very useful method for generating specific enolates. 36 The starting enones are often
readily available and the position of the double bond in the enone determines the
structure of the resulting enolate. For acyclic enones, the TMS-Cl trapping of enolates
generated by conjugate reduction gives a silyl enol ether having a composition that
37
reflects the conformation of the enone. (See Section 2.2.1 of Part A to review enone
conformation.)
CH(CH ) )
3 2
CH 2 1) L-Selectride CH CH(CH 3 2
O N 3 OTMS
CH (CH ) 2) TMS-Cl, Et 3
3
2 5
s-trans CH (CH )
3
2 5
69%; 170:1 Z:E
CH (CH )
)
1) Li, NH 3
CH(CH 3 2 3 2 3
)
CH (CH ) O 2) TMS-Cl, Et N CH(CH 3 2
3
2 3
s-cis 3 TMSO
82% 300:1 E:Z
Trimethylsilyl enol ethers can also be prepared by 1,4-reduction of enones using
38
silanes as reductants. Several effective catalysts have been found, of which the most
versatile appears to be a Pt complex of divinyltetramethyldisiloxane. 39 This catalyst
gives good yields of substituted silyl enol ethers (e.g., Scheme 1.2, Entry 7).
32 H. O. House, L. J. Czuba, M. Gall, and H. D. Olmstead, J. Org. Chem., 34, 2324 (1969); R. D. Miller
and D. R. McKean, Synthesis, 730 (1979).
33 J. Orban, J. V. Turner, and B. Twitchin, Tetrahedron Lett., 25, 5099 (1984).
34
H. Emde, A. Goetz, K. Hofmann, and G. Simchen, Liebigs Ann. Chem., 1643 (1981); see also E. J.
Corey, H. Cho, C. Ruecker, and D. Hua, Tetrahedron Lett., 3455 (1981).
35 E. J. Corey and A. W. Gross, Tetrahedron Lett., 25, 495 (1984).
36 For a review of -enone reduction, see D. Caine, Org. React., 23, 1 (1976).
37
A. R. Chamberlin and S. H. Reich, J. Am. Chem. Soc., 107, 1440 (1985).
38 I. Ojima and T. Kogure, Organometallics, 1, 1390 (1982); T. H. Chan and G. Z. Zheng, Tetrahedron
Lett., 34, 3095 (1993); D. E. Cane and M. Tandon, Tetrahedron Lett., 35, 5351 (1994).
39
C. R. Johnson and R. K Raheja, J. Org. Chem., 59, 2287 (1994).