Page 47 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 47
19
SECTION 1.1
Generation and
Properties of Enolates
and Other
Stabilized Carbanions
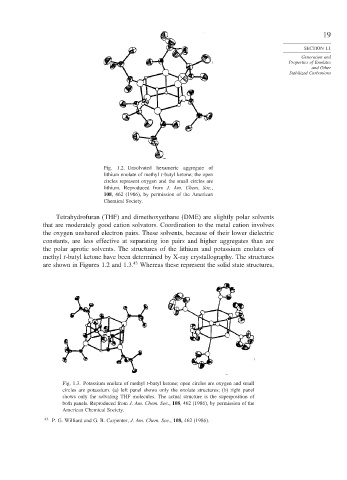
Fig. 1.2. Unsolvated hexameric aggregate of
lithium enolate of methyl t-butyl ketone; the open
circles represent oxygen and the small circles are
lithium. Reproduced from J. Am. Chem. Soc.,
108, 462 (1986), by permission of the American
Chemical Society.
Tetrahydrofuran (THF) and dimethoxyethane (DME) are slightly polar solvents
that are moderately good cation solvators. Coordination to the metal cation involves
the oxygen unshared electron pairs. These solvents, because of their lower dielectric
constants, are less effective at separating ion pairs and higher aggregates than are
the polar aprotic solvents. The structures of the lithium and potassium enolates of
methyl t-butyl ketone have been determined by X-ray crystallography. The structures
are shown in Figures 1.2 and 1.3. 43 Whereas these represent the solid state structures,
Fig. 1.3. Potassium enolate of methyl t-butyl ketone; open circles are oxygen and small
circles are potassium. (a) left panel shows only the enolate structures; (b) right panel
shows only the solvating THF molecules. The actual structure is the superposition of
both panels. Reproduced from J. Am. Chem. Soc., 108, 462 (1986), by permission of the
American Chemical Society.
43
P. G. Williard and G. B. Carpenter, J. Am. Chem. Soc., 108, 462 (1986).