Page 38 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 38
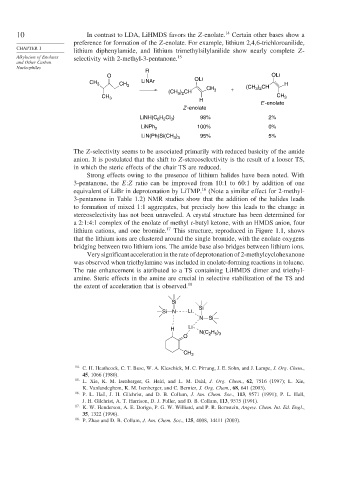
10 In contrast to LDA, LiHMDS favors the Z-enolate. 14 Certain other bases show a
preference for formation of the Z-enolate. For example, lithium 2,4,6-trichloroanilide,
CHAPTER 1
lithium diphenylamide, and lithium trimethylsilylanilide show nearly complete Z-
Alkylation of Enolates selectivity with 2-methyl-3-pentanone. 15
and Other Carbon
Nucleophiles
R
O OLi
CH 3 CH 3 LiNAr OLi (CH ) CH H
(CH ) CH CH 3 + 3 2
3 2
CH 3 CH 3
H
E -enolate
Z -enolate
LiNH(C 6 H 2 Cl 3 ) 98% 2%
LiNPh 2 100% 0%
95% 5%
LiN(Ph)Si(CH 3 ) 3
The Z-selectivity seems to be associated primarily with reduced basicity of the amide
anion. It is postulated that the shift to Z-stereoselectivity is the result of a looser TS,
in which the steric effects of the chair TS are reduced.
Strong effects owing to the presence of lithium halides have been noted. With
3-pentanone, the E:Z ratio can be improved from 10:1 to 60:1 by addition of one
equivalent of LiBr in deprotonation by LiTMP. 16 (Note a similar effect for 2-methyl-
3-pentanone in Table 1.2) NMR studies show that the addition of the halides leads
to formation of mixed 1:1 aggregates, but precisely how this leads to the change in
stereoselectivity has not been unraveled. A crystal structure has been determined for
a 2:1:4:1 complex of the enolate of methyl t-butyl ketone, with an HMDS anion, four
lithium cations, and one bromide. 17 This structure, reproduced in Figure 1.1, shows
that the lithium ions are clustered around the single bromide, with the enolate oxygens
bridging between two lithium ions. The amide base also bridges between lithium ions.
Very significant acceleration in the rate of deprotonation of 2-methylcyclohexanone
was observed when triethylamine was included in enolate-forming reactions in toluene.
The rate enhancement is attributed to a TS containing LiHMDS dimer and triethyl-
amine. Steric effects in the amine are crucial in selective stabilization of the TS and
the extent of acceleration that is observed. 18
Si
Si
Si N Li
N Si
H Li
N(C H )
2 5 3
O
CH 3
14 C. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lampe, J. Org. Chem.,
45, 1066 (1980).
15
L. Xie, K. M. Isenberger, G. Held, and L. M. Dahl, J. Org. Chem., 62, 7516 (1997); L. Xie,
K. Vanlandeghem, K. M. Isenberger, and C. Bernier, J. Org. Chem., 68, 641 (2003).
16
P. L. Hall, J. H. Gilchrist, and D. B. Collum, J. Am. Chem. Soc., 113, 9571 (1991); P. L. Hall,
J. H. Gilchrist, A. T. Harrison, D. J. Fuller, and D. B. Collum, 113, 9575 (1991).
17 K. W. Henderson, A. E. Dorigo, P. G. W. Williard, and P. R. Bernstein, Angew. Chem. Int. Ed. Engl.,
35, 1322 (1996).
18
P. Zhao and D. B. Collum, J. Am. Chem. Soc., 125, 4008, 14411 (2003).