Page 36 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 36
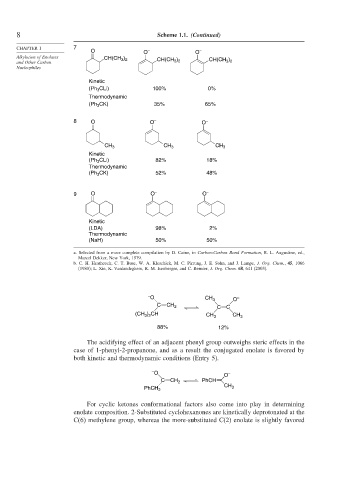
8 Scheme 1.1. (Continued)
CHAPTER 1 7
O O – O –
Alkylation of Enolates
CH(CH 3 ) 2 CH(CH )
and Other Carbon CH(CH 3 ) 2 3 2
Nucleophiles
Kinetic
(Ph 3 CLi) 100% 0%
Thermodynamic
(Ph 3 CK) 35% 65%
8 O O – O –
CH 3 CH 3 CH 3
Kinetic
(Ph 3 CLi) 82% 18%
Thermodynamic
(Ph 3 CK) 52% 48%
9 O O – O –
Kinetic
(LDA) 98% 2%
Thermodynamic
(NaH) 50% 50%
a. Selected from a more complete compilation by D. Caine, in Carbon-Carbon Bond Formation, R. L. Augustine, ed.,
Marcel Dekker, New York, 1979.
b. C. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lampe, J. Org. Chem., 45, 1066
(1980); L. Xie, K. Vanlandeghem, K. M. Isenberger, and C. Bernier, J. Org. Chem. 68, 641 (2003).
– O CH 3 O –
C
CH 2
C C
) CH
(CH 3 2 CH 3 CH 3
88% 12%
The acidifying effect of an adjacent phenyl group outweighs steric effects in the
case of 1-phenyl-2-propanone, and as a result the conjugated enolate is favored by
both kinetic and thermodynamic conditions (Entry 5).
– O O –
C CH 2 PhCH
PhCH 2 CH 3
For cyclic ketones conformational factors also come into play in determining
enolate composition. 2-Substituted cyclohexanones are kinetically deprotonated at the
C(6) methylene group, whereas the more-substituted C(2) enolate is slightly favored