Page 58 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 58
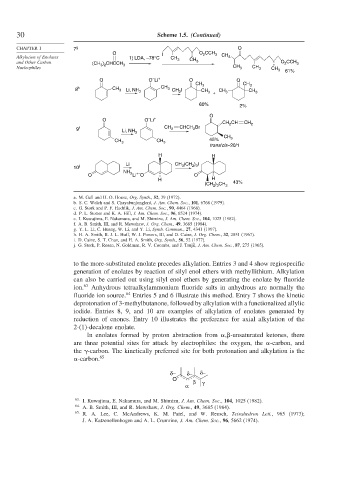
30 Scheme 1.5. (Continued)
CHAPTER 1 7 g O
O I O CCH
Alkylation of Enolates 1) LDA, –78°C CH 3 CH 2 3 CH 3
and Other Carbon (CH ) CHCCH 3 3 O 2 CCH 3
3 2
Nucleophiles CH 3 CH 3 CH 3 61%
–
O O Li + O O
CH 3 CH 3
8 h CH 3 Li, NH CH 3 CH I CH
3 3 CH 3 + 3 CH 3
60% 2%
O
–
O O Li +
CH CH CH 2
2
2
9 i CH 2 CHCH Br
Li, NH 3
CH
CH 45% 3
CH 3 3
trans/cis~20/1
H H
Li CH (CH ) I
10 j 3 2 3
NH
+–
O 3 Li O O
H H
(CH ) CH 3 43%
2 3
a. M. Gall and H. O. House, Org. Synth., 52, 39 (1972).
b. S. C. Welch and S. Chayabunjonglerd, J. Am. Chem. Soc., 101, 6768 (1979).
c. G. Stork and P. F. Hudrlik, J. Am. Chem. Soc., 90, 4464 (1968).
d. P. L. Stotter and K. A. Hill, J. Am. Chem. Soc., 96, 6524 (1974).
e. I. Kuwajima, E. Nakamura, and M. Shimizu, J. Am. Chem. Soc., 104, 1025 (1982).
f. A. B. Smith, III, and R. Mewshaw, J. Org. Chem., 49, 3685 (1984).
g. Y. L. Li, C. Huang, W. Li, and Y. Li, Synth. Commun., 27, 4341 (1997).
h. H. A. Smith, B. J. L. Huff, W. J. Powers, III, and D. Caine, J. Org. Chem., 32, 2851 (1967).
i. D. Caine, S. T. Chao, and H. A. Smith, Org. Synth., 56, 52 (1977).
j. G. Stork, P. Rosen, N. Goldman, R. V. Coombs, and J. Tsujii, J. Am. Chem. Soc., 87, 275 (1965).
to the more-substituted enolate precedes alkylation. Entries 3 and 4 show regiospecific
generation of enolates by reaction of silyl enol ethers with methyllithium. Alkylation
can also be carried out using silyl enol ethers by generating the enolate by fluoride
ion. 63 Anhydrous tetraalkylammonium fluoride salts in anhydrous are normally the
64
fluoride ion source. Entries 5 and 6 illustrate this method. Entry 7 shows the kinetic
deprotonation of 3-methylbutanone, followed by alkylation with a functionalized allylic
iodide. Entries 8, 9, and 10 are examples of alkylation of enolates generated by
reduction of enones. Entry 10 illustrates the preference for axial alkylation of the
2-(1)-decalone enolate.
In enolates formed by proton abstraction from , -unsaturated ketones, there
are three potential sites for attack by electrophiles: the oxygen, the -carbon, and
the -carbon. The kinetically preferred site for both protonation and alkylation is the
-carbon. 65
δ– δ− δ−
O β γ
α
63 I. Kuwajima, E. Nakamura, and M. Shimizu, J. Am. Chem. Soc., 104, 1025 (1982).
64 A. B. Smith, III, and R. Mewshaw, J. Org. Chem., 49, 3685 (1984).
65
R. A. Lee, C. McAndrews, K. M. Patel, and W. Reusch, Tetrahedron Lett., 965 (1973);
J. A. Katzenellenbogen and A. L. Crumrine, J. Am. Chem. Soc., 96, 5662 (1974).