Page 56 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 56
28
CHAPTER 1
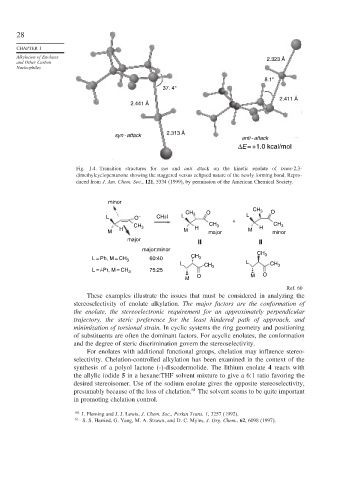
Alkylation of Enolates 2.323 Å
and Other Carbon
Nucleophiles
8.1°
37. 4°
2.411 Å
2.441 Å
2.313 Å
syn - attack
anti - attack
Δ E = +1.0 kcal/mol
Fig. 1.4. Transition structures for syn and anti attack on the kinetic enolate of trans-2,3-
dimethylcyclopentanone showing the staggered versus eclipsed nature of the newly forming bond. Repro-
duced from J. Am. Chem. Soc., 121, 5334 (1999), by permission of the American Chemical Society.
minor
CH 3 O
CH 3 O
L O – CH3I L + L
CH CH 3 CH 3
H 3 H M H
M M major minor
major
major:minor CH
L = Ph, M = CH 3 60:40 CH 3 3
L CH L CH 3
L = i-Pr, M = CH 75:25 3
3
M O M O
Ref. 60
These examples illustrate the issues that must be considered in analyzing the
stereoselectivity of enolate alkylation. The major factors are the conformation of
the enolate, the stereoelectronic requirement for an approximately perpendicular
trajectory, the steric preference for the least hindered path of approach, and
minimization of torsional strain. In cyclic systems the ring geometry and positioning
of substituents are often the dominant factors. For acyclic enolates, the conformation
and the degree of steric discrimination govern the stereoselectivity.
For enolates with additional functional groups, chelation may influence stereo-
selectivity. Chelation-controlled alkylation has been examined in the context of the
synthesis of a polyol lactone (-)-discodermolide. The lithium enolate 4 reacts with
the allylic iodide 5 in a hexane:THF solvent mixture to give a 6:1 ratio favoring the
desired stereoisomer. Use of the sodium enolate gives the opposite stereoselectivity,
61
presumably because of the loss of chelation. The solvent seems to be quite important
in promoting chelation control.
60 I. Fleming and J. J. Lewis, J. Chem. Soc., Perkin Trans. 1, 3257 (1992).
61
S. S. Harried, G. Yang, M. A. Strawn, and D. C. Myles, J. Org. Chem., 62, 6098 (1997).