Page 65 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 65
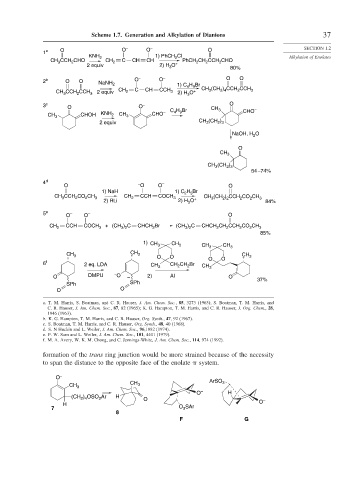
Scheme 1.7. Generation and Alkylation of Dianions 37
1 a O O – O – O SECTION 1.2
KNH 2 1) PhCH 2 Cl Alkylation of Enolates
CH CCH CHO CH 2 C CH CH PhCH CH CCH CHO
3
2
2
2
2
2 equiv 2) H O + 80%
3
2 b O O O – O – O O
NaNH 2 1) C H Br
4 9
CH C CH CCH CH (CH ) CCH CCH 3
2 4
2
3
2 equiv 2 3 2) H O +
CH 3 CCH 2 CCH 3 3
3 c O O – C H Br CH 3 O –
CHOH KNH 2 CH CHO – 4 9 CHO
CH 3 3
3
2 equiv CH (CH )
2 3
NaOH, H O
2
O
CH 3
CH (CH )
3
2 3
54 –74%
4 d – –
O O O O
1) NaH 1) C H Br
2 5
CCH CO CH CCH
3
CH 3 2 2 3 CH 2 COCH 3 + CH (CH 2 ) 2 CCH 2 CO 2 CH 3
2) RLi 2) H O 84%
3
5 e O – O – O
CH 2 CCH COCH 3 + (CH 3 ) 2 C CHCH 2 Br (CH 3 ) 2 C CHCH 2 CH 2 CCH 2 CO 2 CH 3
85%
1) CH 3 CH 3 CH 3 CH 3
CH 3 CH 3 O O CH 3
6 f 2 eq. LDA CH 3 CH 2 CH 2 Br CH O O
3
O DMPU – O – 2) Al O 37%
SPh SPh
O O
a. T. M. Harris, S. Boatman, and C. R. Hauser, J. Am. Chem. Soc., 85, 3273 (1963); S. Boatman, T. M. Harris, and
C. R. Hauser, J. Am. Chem. Soc., 87, 82 (1965); K. G. Hampton, T. M. Harris, and C. R. Hauser, J. Org. Chem., 28,
1946 (1963).
b. K. G. Hampton, T. M. Harris, and C. R. Hauser, Org. Synth., 47, 92 (1967).
c. S. Boatman, T. M. Harris, and C. R. Hauser, Org. Synth., 48, 40 (1968).
d. S. N Huckin and L. Weiler, J. Am. Chem. Soc., 96,1082 (1974).
e. F. W. Sum and L. Weiler, J. Am. Chem. Soc., 101, 4401 (1979).
f. M. A. Avery, W. K. M. Chong, and C. Jennings-White, J. Am. Chem. Soc., 114, 974 (1992).
formation of the trans ring junction would be more strained because of the necessity
to span the distance to the opposite face of the enolate system.
O –
ArSO
CH 3 CH 3 3
O – H
(CH ) OSO Ar H O –
2 4
2
H O
7 O SAr
3
8
F G