Page 66 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 66
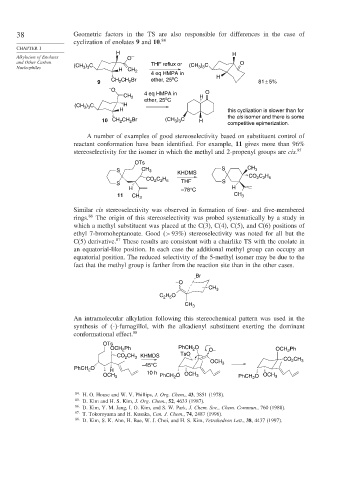
38 Geometric factors in the TS are also responsible for differences in the case of
cyclization of enolates 9 and 10. 84
CHAPTER 1
H H
Alkylation of Enolates O –
and Other Carbon THF reflux or (CH 3 ) 3 C O
3 3
Nucleophiles (CH ) C H
CH 2
4 eq HMPA in
o
CH CH Br ether, 25 C H
9 2 2 81 ± 5%
– O
4 eq HMPA in O
CH 2 o H
ether, 25 C
(CH ) C H
3 3
H this cyclization is slower than for
the cis isomer and there is some
) C
10 CH 2 CH Br (CH 3 3 H
2
competitive epimerization.
A number of examples of good stereoselectivity based on substituent control of
reactant conformation have been identified. For example, 11 gives more than 96%
stereoselectivity for the isomer in which the methyl and 2-propenyl groups are cis. 85
OTs
S CH 3 S CH 3
KHDMS CO C H
CO C H 2 2 5
2 2 5
S THF S
H –78°C H
11 CH 3 CH 3
Similar cis stereoselectivity was observed in formation of four- and five-membered
rings. 86 The origin of this stereoselectivity was probed systematically by a study in
which a methyl substituent was placed at the C(3), C(4), C(5), and C(6) positions of
ethyl 7-bromoheptanoate. Good >93% stereoselectivity was noted for all but the
C(5) derivative. 87 These results are consistent with a chairlike TS with the enolate in
an equatorial-like position. In each case the additional methyl group can occupy an
equatorial position. The reduced selectivity of the 5-methyl isomer may be due to the
fact that the methyl group is farther from the reaction site than in the other cases.
Br
–O
CH 3
C H O
2 5
CH 3
An intramolecular alkylation following this stereochemical pattern was used in the
synthesis of (-)-fumagillol, with the alkadienyl substituent exerting the dominant
conformational effect. 88
OTs
OCH Ph PhCH O O– OCH Ph
2
2
2
CO CH 3 KHMDS TsO
2
OCH 3 CO 2 CH 3
–45°C
PhCH O H
2
OCH 3 10 h PhCH 2 O OCH 3 PhCH O OCH 3
2
84 H. O. House and W. V. Phillips, J. Org. Chem., 43, 3851 (1978).
85
D. Kim and H. S. Kim, J. Org. Chem., 52, 4633 (1987).
86
D. Kim, Y. M. Jang, I. O. Kim, and S. W. Park, J. Chem. Soc., Chem. Commun., 760 (1988).
87 T. Tokoroyama and H. Kusaka, Can. J. Chem., 74, 2487 (1996).
88
D. Kim, S. K. Ahn, H. Bae, W. J. Choi, and H. S. Kim, Tetrahedron Lett., 38, 4437 (1997).