Page 68 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 68
40 Scheme 1.8. (Continued)
CHAPTER 1 7 g
(CH 2 ) 2 I
Alkylation of Enolates O
and Other Carbon O H O
Nucleophiles CH 3 LiHMDS
O CH 3
THF-HMPA H
C 2 H 5 O 2 CCH 2
CH 3 O 2 C
56% 2
8 h Ar 2 O Ar
OH 1) (C 2 H 5 O) 2 PCl
CO 2 C(CH 3 ) 3
CH 3 (CH 2 ) 3 N 2) LiHMDS CH 3 (CH 2 ) 3 N
CO 2 C(CH 3 ) 3
Ar 1 Ar 1
O
Ar 1 = OTBDMS 2 O
Ar 70% on
2-kg scale
CH 3
OCH 3
a. A. Srikrishna, G. V. R. Sharma, S. Danieldoss, and P. Hemamalini, J. Chem. Soc., Perkin Trans. 1, 1305 (1996).
b. E. Piers, W. de Waal, and R. W. Britton, J. Am. Chem. Soc., 93, 5113 (1971).
c. D. Kim, S. Kim, J.-J. Lee, and H. S. Kim, Tetrahedron Lett., 31, 4027 (1990).
d. D. Kim, J. I. Lim, K. J. Shin, and H. S. Kim, Tetrahedron Lett., 34, 6557 (1993).
e. J. Lee and J. Hong, J. Org. Chem., 69, 6433 (2004).
f. F.-D. Boyer and P.-H. Ducrot, Eur. J. Org. Chem., 1201 (1999).
g. S. Danishefsky, K. Vaughan, R. C. Gadwood, and K. Tsuzuki, J. Am. Chem. Soc., 102, 4262 (1980).
h. Z. J. Song, M. Zhao, R. Desmond, P. Devine, D. M. Tschaen, R. Tillyer, L.Frey, R. Heid, F. Xu;, B. Foster, J. Li,
R. Reamer, R. Volante, E. J. Grabowski, U. H. Dolling, P. J. Reider, S. Okada, Y. Kato and E. Mano, J. Org. Chem.,
64, 9658 (1999).
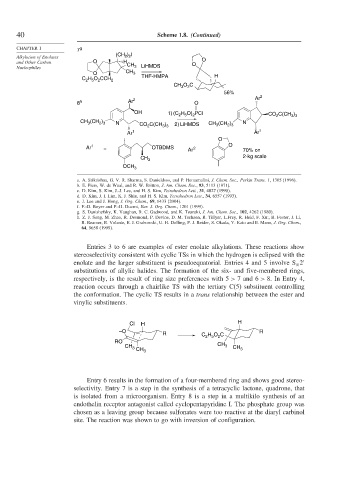
Entries 3 to 6 are examples of ester enolate alkylations. These reactions show
stereoselectivity consistent with cyclic TSs in which the hydrogen is eclipsed with the
enolate and the larger substituent is pseudoequatorial. Entries 4 and 5 involve S 2
N
substitutions of allylic halides. The formation of the six- and five-membered rings,
respectively, is the result of ring size preferences with 5 > 7 and 6 > 8. In Entry 4,
reaction occurs through a chairlike TS with the tertiary C(5) substituent controlling
the conformation. The cyclic TS results in a trans relationship between the ester and
vinylic substituents.
Cl H H
–O R
R C H O C
2
2 5
RO
CH 3 CH 3 CH
CH 3 3
Entry 6 results in the formation of a four-membered ring and shows good stereo-
selectivity. Entry 7 is a step in the synthesis of a tetracyclic lactone, quadrone, that
is isolated from a microorganism. Entry 8 is a step in a multikilo synthesis of an
endothelin receptor antagonist called cyclopentapyridine I. The phosphate group was
chosen as a leaving group because sulfonates were too reactive at the diaryl carbinol
site. The reaction was shown to go with inversion of configuration.