Page 71 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 71
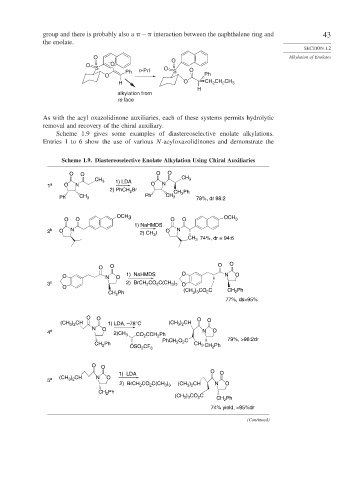
group and there is probably also a − interaction between the naphthalene ring and 43
the enolate.
SECTION 1.2
O Alkylation of Enolates
– O
O O
S O
Ph n-PrI S O
O Ph
H O CH CH CH 3
2
2
H
alkylation from
re face
As with the acyl oxazolidinone auxiliaries, each of these systems permits hydrolytic
removal and recovery of the chiral auxiliary.
Scheme 1.9 gives some examples of diastereoselective enolate alkylations.
Entries 1 to 6 show the use of various N-acyloxazolidinones and demonstrate the
Scheme 1.9. Diastereoselective Enolate Alkylation Using Chiral Auxiliaries
O O O O
CH 3
CH 3 1) LDA
1 a O N O N
2) PhCH 2 Br CH 2 Ph
Ph CH 3 Ph CH 3 78%, dr 98:2
OCH 3
O O O O OCH 3
1) NaHMDS
2 b O N 2) CH 3 I O N
CH 3 74%, dr = 94:6
O O O O
O N O 1) NaHMDS O N O
3 c 2) BrCH 2 CO 2 C(CH 3 ) 3 O
O
CH 2 Ph (CH ) 3 CO 2 C CH 2 Ph
3
77%, ds>95%
O O O
(CH 3 ) 2 CH 1) LDA, –78°C (CH 3 ) 2 CH O
N O
4 d 2)CH 3 CO 2 CCH 2 Ph N O
PhCH 2 O 2 C 79%, >98:2dr
CH 2 Ph CH 3 CH 2 Ph
OSO 2 CF 3
O O
O
1) LDA O
5 e (CH 3 ) 2 CH N O
2) BrCH 2 CO 2 C(CH 3 ) 3 (CH 3 ) 2 CH N O
CH 2 Ph
(CH 3 ) 3 CO 2 C
CH 2 Ph
74% yield, >95%dr
(Continued)