Page 82 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 82
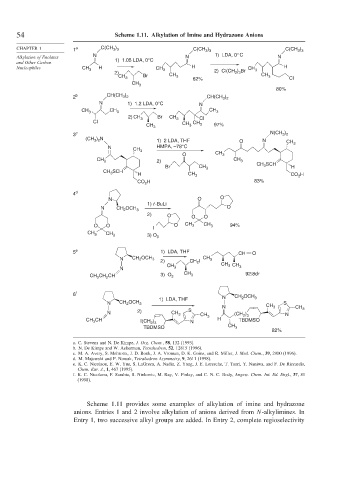
54 Scheme 1.11. Alkylation of Imine and Hydrazone Anions
CHAPTER 1 1 a C(CH ) C(CH ) C(CH )
3 3
3 3
3 3
N 1) LDA, 0° C
Alkylation of Enolates N N
and Other Carbon 1) 1.05 LDA, 0°C
Nucleophiles CH 3 H CH H CH 3 H
) Br
2) 3 CH 2) Cl(CH 2 3 CH
CH 3 Br 3 62% 3 Cl
CH 3
80%
2 b CH(CH ) CH(CH )
3 2
3 2
N 1) 1.2 LDA, 0°C N
CH 3 CH 3 CH 3
2) CH 3 Br CH 3 Cl
Cl CH CH
CH 3 3 3 97%
3 2
3 c N(CH )
) N
(CH 3 2
1) 2 LDA, THF O N CH 3
N CH 3 HMPA, –78°C
O CH 3
CH 3 2) CH 3
Br CH 3 CH SCH H
3
SCH
CH 3
H CH 3 CO 2 H
CO H 83%
2
4 d
N O O
1) t -BuLi
N CH OCH 3 O
2
2) O O O
O O O CH 3 CH 3 94%
I
CH 3
CH 3
3) O 3
5 e 1) LDA, THF CH O
N CH 2 OCH 3 CH 3
2) CH 2 I
CH CH CH
3
N 3 3
CH CH CH 3) O 3 CH 3 92:8dr
2
3
6 f CH OCH
1) LDA, THF N 2 3
N CH 2 OCH 3 S
N CH 3 CH 3
N 2) CH 3 S CH (CH ) N
2 3
CH CH I(CH 2 3 N 3 H TBDMSO
)
3
TBDMSO CH 3
82%
a. C. Stevens and N. De Kimpe, J. Org. Chem., 58, 132 (1993).
b. N. De Kimpe and W. Aelterman, Tetrahedron, 52, 12815 (1996).
c. M. A. Avery, S. Mehrotra, J. D. Bonk, J. A. Vroman, D. K. Goins, and R. Miller, J. Med. Chem., 39, 2900 (1996).
d. M. Majewski and P. Nowak, Tetrahedron Asymmetry, 9, 2611 (1998).
e. K. C. Nicolaou, E. W. Yue, S. LaGreca, A. Nadin, Z. Yang, J. E. Leresche, T. Tsuri, Y. Naniwa, and F. De Riccardis,
Chem. Eur. J., 1, 467 (1995).
f. K. C. Nicolaou, F. Sarabia, S. Ninkovic, M. Ray, V. Finlay, and C. N. C. Body, Angew. Chem. Int. Ed. Engl., 37,81
(1998).
Scheme 1.11 provides some examples of alkylation of imine and hydrazone
anions. Entries 1 and 2 involve alkylation of anions derived from N-alkylimines. In
Entry 1, two successive alkyl groups are added. In Entry 2, complete regioselectivity