Page 85 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 85
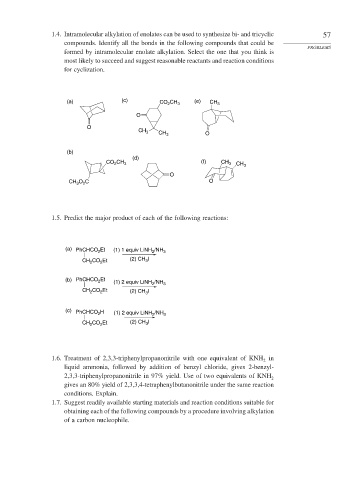
1.4. Intramolecular alkylation of enolates can be used to synthesize bi- and tricyclic 57
compounds. Identify all the bonds in the following compounds that could be
PROBLEMS
formed by intramolecular enolate alkylation. Select the one that you think is
most likely to succeed and suggest reasonable reactants and reaction conditions
for cyclization.
(a) (c) CO CH 3 (e) CH 3
2
O
O
CH 3 CH 3 O
(b)
(d)
CO CH 3 (f) CH 3 CH 3
2
O
O C O
CH 3 2
1.5. Predict the major product of each of the following reactions:
Et (1) 1 equiv LiNH /NH
(a) PhCHCO 2 2 3
CH CO Et (2) CH I
3
2
2
(b) PhCHCO 2 Et
(1) 2 equiv LiNH /NH 3
2
CH CO Et (2) CH I
2
2
3
(c) PhCHCO H (1) 2 equiv LiNH /NH 3
2
2
CH CO Et (2) CH I
3
2
2
1.6. Treatment of 2,3,3-triphenylpropanonitrile with one equivalent of KNH in
2
liquid ammonia, followed by addition of benzyl chloride, gives 2-benzyl-
2,3,3-triphenylpropanonitrile in 97% yield. Use of two equivalents of KNH 2
gives an 80% yield of 2,3,3,4-tetraphenylbutanonitrile under the same reaction
conditions. Explain.
1.7. Suggest readily available starting materials and reaction conditions suitable for
obtaining each of the following compounds by a procedure involving alkylation
of a carbon nucleophile.