Page 88 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 88
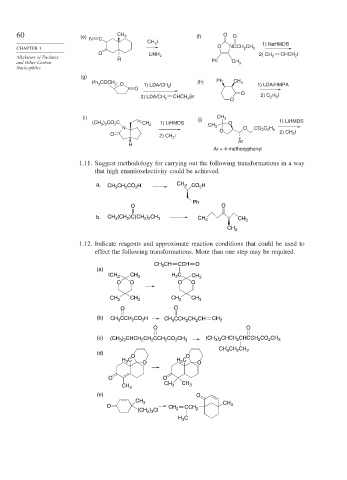
60 (e) CH 3 (f) O O
C
N
CH I 1) NaHMDS
3
CHAPTER 1 O NCCH 2 CH 3
O LiNH 2) CH CHCH I
Alkylation of Enolates H 2 2 2
and Other Carbon Ph CH 3
Nucleophiles
(g)
COCH (h) Ph CH 3
Ph 3 2 O 1) LDA/CH I 1) LDA/HMPA
O 3
O
H I
CHCH Br 2) C 2 5
2) LDA/CH 2 2 O
(i) (j) CH 3
(CH ) CO C CH 2 1) LiHMDS CH O 1) LiHMDS
2
3 3
CH
N 3 3 O CO 2 2 5
C H
3
O 2) CH I O 2) CH I
3
Ar
H
Ar = 4-methoxyphenyl
1.11. Suggest methodology for carrying out the following transformations in a way
that high enantioselectivity could be achieved.
a. CH CH CO H CH 3 CO H
2
2
2
3
Ph
O O
b. CH (CH )C(CH ) CH 3 CH 3 CH 3
2 2
3
2
CH 3
1.12. Indicate reagents and approximate reaction conditions that could be used to
effect the following transformations. More than one step may be required.
CH CH CCH O
3
(a)
ICH 2 CH 3 H C CH 3
2
O O O O
CH 3 CH 3 CH 3 CH 3
O O
(b) CH CCH CO H CH CCH CH CH CH 2
2
3
3
2
2
2
O O
(c) (CH ) CHCH CH CCH CO CH 3 (CH ) CHCH CHCCH CO CH 3
2
3 2
2
2
2
3 2
2
2
2
CH CH CH 2
2
3
(d)
O O
H C O H C O
3
3
O O
CH 3 CH 3 CH 3
(e) O
CH 3 CH
O CH 2 CCH 2 3
) Cl
(CH 2 3
H C
3