Page 92 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 92
64
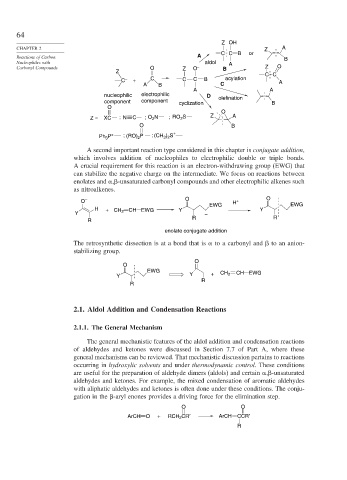
Z OH
CHAPTER 2 Z A
Reactions of Carbon A CC B or B
Nucleophiles with aldol A
Carbonyl Compounds O Z O – B Z O
Z
CC
C – + C C C B acylation A
A B C
A A
nucleophilic electrophilic D olefination
component component cyclization B
O
O
Z = XC ; N C ; O N ; RO 2 S Z A
2
O B
Ph P + ; (RO) 2 P ; (CH ) S +
3 2
3
A second important reaction type considered in this chapter is conjugate addition,
which involves addition of nucleophiles to electrophilic double or triple bonds.
A crucial requirement for this reaction is an electron-withdrawing group (EWG) that
can stabilize the negative charge on the intermediate. We focus on reactions between
enolates and , -unsaturated carbonyl compounds and other electrophilic alkenes such
as nitroalkenes.
O – O H + O
EWG EWG
H + CH 2 CH EWG Y Y
Y –
R R
R
enolate conjugate addition
The retrosynthetic dissection is at a bond that is to a carbonyl and to an anion-
stabilizing group.
O
O
EWG CH 2 CH EWG
Y Y +
R
R
2.1. Aldol Addition and Condensation Reactions
2.1.1. The General Mechanism
The general mechanistic features of the aldol addition and condensation reactions
of aldehydes and ketones were discussed in Section 7.7 of Part A, where these
general mechanisms can be reviewed. That mechanistic discussion pertains to reactions
occurring in hydroxylic solvents and under thermodynamic control. These conditions
are useful for the preparation of aldehyde dimers (aldols) and certain , -unsaturated
aldehydes and ketones. For example, the mixed condensation of aromatic aldehydes
with aliphatic aldehydes and ketones is often done under these conditions. The conju-
gation in the -aryl enones provides a driving force for the elimination step.
O O
ArCH O + RCH 2 CR′ ArCH CCR′
R