Page 117 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 117
selection of the optimum enolate system requires analyses of the individual cases. 74 89
Often, one of the available reactant systems proves to be superior. 75 Sometimes a
remote structural feature strongly influences the stereoselectivity. 76 The issues that SECTION 2.1
have to be addressed in specific cases include the structure of the reactants, including its Aldol Addition and
Condensation Reactions
configuration and potential sites for chelation; the organization of the TS (cyclic, open,
or chelated); and the steric, electronic, and polar factors affecting the facial selectivity.
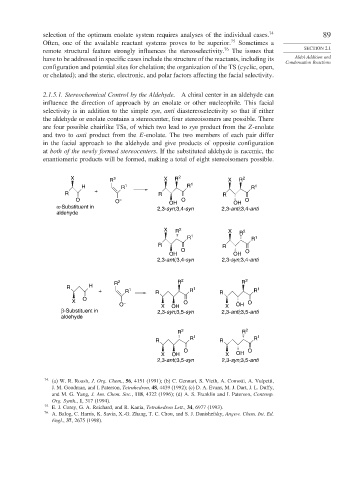
2.1.5.1. Stereochemical Control by the Aldehyde. A chiral center in an aldehyde can
influence the direction of approach by an enolate or other nucleophile. This facial
selectivity is in addition to the simple syn, anti diastereoselectivity so that if either
the aldehyde or enolate contains a stereocenter, four stereoisomers are possible. There
are four possible chairlike TSs, of which two lead to syn product from the Z-enolate
and two to anti product from the E-enolate. The two members of each pair differ
in the facial approach to the aldehyde and give products of opposite configuration
at both of the newly formed stereocenters. If the substituted aldehyde is racemic, the
enantiomeric products will be formed, making a total of eight stereoisomers possible.
X R 2 X R 2 X R 2
H R 1 R 1 R 1
+
R R R
O O – OH O OH O
α-Substituent in 2,3-syn;3,4-syn 2,3-anti;3,4-anti
aldehyde
X R 2 X R 2
R 1 R 1
R R
O O
OH OH
2,3-anti;3,4-syn 2,3-syn;3,4-anti
R 2 R 2 R 2
R H 1 1
+ R 1 R R R R
O
X – O O
O X OH X OH
β-Substituent in 2,3-syn;3,5-syn 2,3-anti;3,5-anti
aldehyde
R 2 R 2
R R 1 R R 1
O O
X OH X OH
2,3-anti;3,5-syn 2,3-syn;3,5-anti
74 (a) W. R. Roush, J. Org. Chem., 56, 4151 (1991); (b) C. Gennari, S. Vieth, A. Comotti, A. Vulpetti,
J. M. Goodman, and I. Paterson, Tetrahedron, 48, 4439 (1992); (c) D. A. Evans, M. J. Dart, J. L. Duffy,
and M. G. Yang, J. Am. Chem. Soc., 118, 4322 (1996); (d) A. S. Franklin and I. Paterson, Contemp.
Org. Synth., 1, 317 (1994).
75 E. J. Corey, G. A. Reichard, and R. Kania, Tetrahedron Lett., 34, 6977 (1993).
76
A. Balog, C. Harris, K. Savin, X.-G. Zhang, T. C. Chou, and S. J. Danishefsky, Angew. Chem. Int. Ed.
Engl., 37, 2675 (1998).