Page 121 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 121
also precluded in Mukaiyama reactions. Chelation control does occur in the Mukaiyama 93
reaction using other Lewis acids. Both - and -alkoxy aldehydes give chelation-
86
controlled products with SnCl and TiCl , but not with BF . If there is an additional SECTION 2.1
4 4 3
substituent on the aldehyde, the chelate establishes a facial preference for the approach Aldol Addition and
Condensation Reactions
of the nucleophile. 87
Ph Ph
Ph
R O Ti Ti R O
R O
O Ph Ph OH
O
H
O O
In each instance, the silyl enol ether approaches anti to the methyl substituent on the
chelate. This results in a 3,4-syn relationship between the hydroxy and alkoxy groups
for -alkoxy aldehydes and a 3,5-anti relationship for -alkoxy aldehydes with the
main chain in the extended conformation.
OH O
PhCH 2 O CH 3 OTMS CH 3
+ TiCl 4
CH O Ph
CH 3 Ph
PhCH 2 O CH 3 97 % 2,3-syn-3,4-syn
3 % 2,3-anti-3,4-anti
Ref. 88
CH 3 OTMS PhCH 2 O OH O
CH O TiCl 4
+ CH 2
PhCH 2 O Ph Ph
CH 3
92:8 3,5-anti:syn
Ref. 84
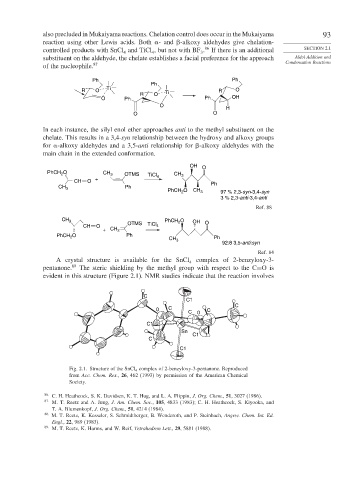
A crystal structure is available for the SnCl 4 complex of 2-benzyloxy-3-
pentanone. 89 The steric shielding by the methyl group with respect to the C=Ois
evident in this structure (Figure 2.1). NMR studies indicate that the reaction involves
C
C1
C
0 C C
C 0
C1
Sn
C1
C
C1
Fig. 2.1. Structure of the SnCl 4 complex of 2-benzyloxy-3-pentanone. Reproduced
from Acc. Chem. Res., 26, 462 (1993) by permission of the American Chemical
Society.
86 C. H. Heathcock, S. K. Davidsen, K. T. Hug, and L. A. Flippin, J. Org. Chem., 51, 3027 (1986).
87
M. T. Reetz and A. Jung, J. Am. Chem. Soc., 105, 4833 (1983); C. H. Heathcock, S. Kiyooka, and
T. A. Blumenkopf, J. Org. Chem., 51, 4214 (1984).
88 M. T. Reetz, K. Kesseler, S. Schmidtberger, B. Wenderoth, and P. Steinbach, Angew. Chem. Int. Ed.
Engl., 22, 989 (1983).
89
M. T. Reetz, K. Harms, and W. Reif, Tetrahedron Lett., 29, 5881 (1988).