Page 116 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 116
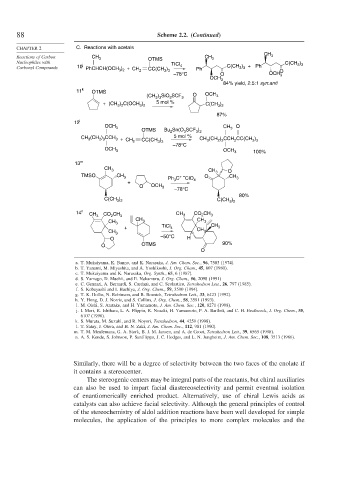
88 Scheme 2.2. (Continued)
CHAPTER 2 C. Reactions with acetals
CH
Reactions of Carbon CH 3 OTMS CH 3 3
Nucleophiles with j TiCl C(CH )
3 3
Carbonyl Compounds 10 PhCHCH(OCH ) + CH CC(CH ) 4 Ph C(CH ) + Ph
3 3
3 2
3 3
2
–78°C O OCH O
3
OCH 3
84% yield, 2.5:1 syn:anti
11 k OTMS
) SiO SCF O OCH
(CH 3 3 3 3 3
+ (CH ) C(OCH ) 5 mol % C(CH )
3 2
3 2
3 2
87%
12 l
OCH CH O
3
OTMS Bu Sn(O SCF ) 3
2
3 2
3
(CH ) CCH 5 mol %
CH 3 2 3 3 + CH CC(CH ) CH (CH ) CCH CC(CH )
2 3
3 3
3
2
2 3 3
–78°C
OCH 3 OCH 3 100%
13 m
CH 3 CH O
TMSO CH 3 Ph C ClO O 3 CH 3
+ –
+ 3 4
O OCH 3
–78°C
80%
C(CH ) C(CH )
3 2
3 2
14 n CH 3 CO CH 3 CH 3 CO CH 3
2
2
CH CH 3 CH 3
3 TiCl CH
+ 4 3
CH 3 CH 3
O –50°C H
O OTMS 90%
O
a. T. Mukaiyama, K. Banno, and K. Narasaka, J. Am. Chem. Soc., 96, 7503 (1974).
b. T. Yanami, M. Miyashita, and A. Yoshikoshi, J. Org. Chem., 45, 607 (1980).
c. T. Mukaiyama and K. Narasaka, Org. Synth., 65, 6 (1987).
d. S. Yamago, D. Machii, and E. Nakamura, J. Org. Chem., 56, 2098 (1991)
e. C. Gennari, A. Bernardi, S. Cardani, and C. Scolastico, Tetrahedron Lett., 26, 797 (1985).
f. S. Kobayashi and I. Hachiya, J. Org. Chem., 59, 3590 (1994).
g. T. K. Hollis, N. Robinson, and B. Bosnich, Tetrahedron Lett., 33, 6423 (1992).
h. Y. Hong, D. J. Norris, and S. Collins, J. Org. Chem., 58, 3591 (1993).
i. M. Oishi, S. Aratake, and H. Yamamoto, J. Am. Chem. Soc., 120, 8271 (1998).
j. I. Mori, K. Ishihara, L. A. Flippin, K. Nozaki, H. Yamamoto, P. A. Bartlett, and C. H. Heathcock, J. Org. Chem., 55,
6107 (1990).
k. S. Murata, M. Suzuki, and R. Noyori, Tetrahedron, 44, 4259 (1998).
l. T. Satay, J. Otera, and H. N. Zaki, J. Am. Chem. Soc., 112, 901 (1990).
m. T. M. Meulemans, G. A. Stork, B. J. M. Jansen, and A. de Groot, Tetrahedron Lett., 39, 6565 (1998).
n. A. S. Kende, S. Johnson, P. Sanfilippo, J. C. Hodges, and L. N. Jungheim, J. Am. Chem. Soc., 108, 3513 (1986).
Similarly, there will be a degree of selectivity between the two faces of the enolate if
it contains a stereocenter.
The stereogenic centers may be integral parts of the reactants, but chiral auxiliaries
can also be used to impart facial diastereoselectivity and permit eventual isolation
of enantiomerically enriched product. Alternatively, use of chiral Lewis acids as
catalysts can also achieve facial selectivity. Although the general principles of control
of the stereochemistry of aldol addition reactions have been well developed for simple
molecules, the application of the principles to more complex molecules and the