Page 120 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 120
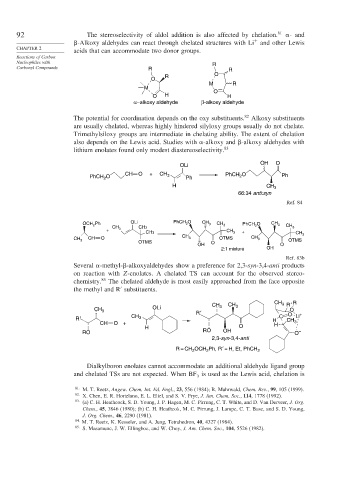
92 The stereoselectivity of aldol addition is also affected by chelation. 81 - and
+
-Alkoxy aldehydes can react through chelated structures with Li and other Lewis
CHAPTER 2
acids that can accommodate two donor groups.
Reactions of Carbon
Nucleophiles with R
Carbonyl Compounds R R
R O
O
M R
M
O
O H H
α−alkoxy aldehyde β-alkoxy aldehyde
The potential for coordination depends on the oxy substituents. 82 Alkoxy substituents
are usually chelated, whereas highly hindered silyloxy groups usually do not chelate.
Trimethylsiloxy groups are intermediate in chelating ability. The extent of chelation
also depends on the Lewis acid. Studies with -alkoxy and -alkoxy aldehydes with
lithium enolates found only modest diastereoselectivity. 83
OH O
OLi
CH O + CH PhCH O
PhCH O 3 Ph 2 Ph
2
H CH 3
66:34 anti:syn
Ref. 84
OCH Ph OLi PhCH O CH 3 CH 3 PhCH O CH
2
2 2 3 CH
CH CH3 3
+ 3 CH +
CH3 3 CH 3
CH O CH 3 OTMS CH
CH 3 3 OTMS
OTMS O
OH O
2:1 mixture OH
Ref. 83b
Several -methyl- -alkoxyaldehydes show a preference for 2,3-syn-3,4-anti products
on reaction with Z-enolates. A chelated TS can account for the observed stereo-
chemistry. 85 The chelated aldehyde is most easily approached from the face opposite
the methyl and R substituents.
CH R
CH CH 3 3 R ′
CH 3 OLi R ′ 3 O
R ′ CH 3 H C O Li +
CH O + H CH 3
H O
RO RO OH O –
2,3-syn-3,4-anti
R = CH OCH Ph, R ′ = H, Et, PhCH 2
2
2
Dialkylboron enolates cannot accommodate an additional aldehyde ligand group
and chelated TSs are not expected. When BF is used as the Lewis acid, chelation is
3
81 M. T. Reetz, Angew. Chem. Int. Ed. Engl., 23, 556 (1984); R. Mahrwald, Chem. Rev., 99, 105 (1999).
82
X. Chen, E. R. Hortelano, E. L. Eliel, and S. V. Frye, J. Am. Chem. Soc., 114, 1778 (1992).
83 (a) C. H. Heathcock, S. D. Young, J. P. Hagen, M. C. Pirrung, C. T. White, and D. Van Derveer, J. Org.
Chem., 45, 3846 (1980); (b) C. H. Heathcok, M. C. Pirrung, J. Lampe, C. T. Buse, and S. D. Young,
J. Org. Chem., 46, 2290 (1981).
84 M. T. Reetz, K. Kesseler, and A. Jung, Tetrahedron, 40, 4327 (1984).
85
S. Masamune, J. W. Ellingboe, and W. Choy, J. Am. Chem. Soc., 104, 5526 (1982).