Page 119 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 119
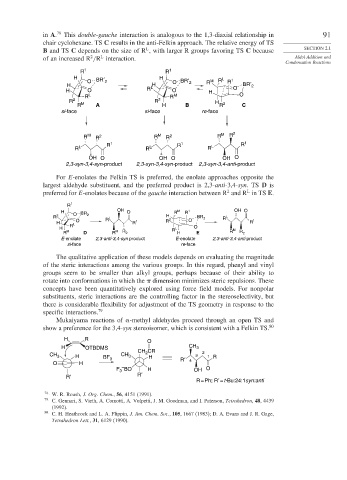
in A. 78 This double-gauche interaction is analogous to the 1,3-diaxial relationship in 91
chair cyclohexane. TS C results in the anti-Felkin approach. The relative energy of TS
L
B and TS C depends on the size of R , with larger R groups favoring TS C because SECTION 2.1
L
2
of an increased R /R interaction. Aldol Addition and
Condensation Reactions
R 1 R 1
H BR′ H L
O 2 O BR′ 2 R M R R 1
H L H BR′ 2
H O R O H O
R L R M O
R 2 R 2 H
R M A H B R 2 C
si-face si-face re-face
R M R 2 R M R 2 R M R 2
R 1 R 1 L R 1
R L R L R
OH O OH O OH O
2,3-syn-3,4-syn-product 2,3-syn-3,4-syn-product 2,3-syn-3,4-anti-product
For E-enolates the Felkin TS is preferred, the enolate approaches opposite the
largest aldehyde substituent, and the preferred product is 2,3-anti-3,4-syn. TS D is
L
2
preferred for E-enolates because of the gauche interaction between R and R in TS E.
R 1
OH OH O
H BR O R M R 1
R 2 O 2 R L H 2 BR 2 R L
H R L O R 1 R O R 1
H R L O M
R M D R M R 2 H E R R 2
E-enolate 2,3-anti-3,4-syn product E-enolate 2,3-anti-3,4-anti product
si-face re-face
The qualitative application of these models depends on evaluating the magnitude
of the steric interactions among the various groups. In this regard, phenyl and vinyl
groups seem to be smaller than alkyl groups, perhaps because of their ability to
rotate into conformations in which the dimension minimizes steric repulsions. These
concepts have been quantitatively explored using force field models. For nonpolar
substituents, steric interactions are the controlling factor in the stereoselectivity, but
there is considerable flexibility for adjustment of the TS geometry in response to the
specific interactions. 79
Mukaiyama reactions of -methyl aldehydes proceed through an open TS and
show a preference for the 3,4-syn stereoisomer, which is consistent with a Felkin TS. 80
H R
O
H OTBDMS CH 3
CH CR 2
CH 3 H BF CH 3 2 H 3 1 R
O H 3 R′ 4
–
F BO H OH O
3
R′ R′
R = Ph; R′ = t-Bu:24:1syn:anti
78
W. R. Roush, J. Org. Chem., 56, 4151 (1991).
79 C. Gennari, S. Vieth, A. Comotti, A. Vulpetti, J. M. Goodman, and I. Paterson, Tetrahedron, 48, 4439
(1992).
80
C. H. Heathcock and L. A. Flippin, J. Am. Chem. Soc., 105, 1667 (1983); D. A. Evans and J. R. Gage,
Tetrahedron Lett., 31, 6129 (1990).