Page 145 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 145
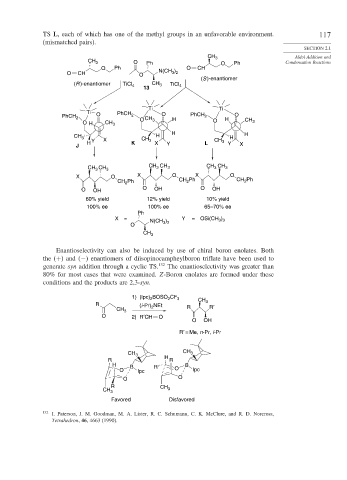
TS L, each of which has one of the methyl groups in an unfavorable environment. 117
(mismatched pairs).
SECTION 2.1
CH 3 Aldol Addition and
CH 3 O Ph O Ph Condensation Reactions
O Ph O CH
O CH O N(CH )
3 2
(S )-enantiomer
(R )-enantiomer TiCl 4 CH 3 TiCl 4
13
Ti Ti
Ti O PhCH O PhCH
PhCH 2 2 CH 2 O
O 3 H O H CH
O H CH 3 3
H H
CH 3 X CH H H
H Y K 3 X Y L CH 3 Y
J X
CH CH 3 CH CH 3 CH CH 3
3
3
3
X O X O X O
2
CH Ph CH 2 Ph CH Ph
2
O OH O OH O OH
60% yield 12% yield 10% yield
100% ee 100% ee 65–70% ee
Ph
X = N(CH ) Y = OSi(CH )
3 3
O 3 2
CH 3
Enantioselectivity can also be induced by use of chiral boron enolates. Both
the (+) and (−) enantiomers of diisopinocampheylboron triflate have been used to
generate syn addition through a cyclic TS. 132 The enantioselectivity was greater than
80% for most cases that were examined. Z-Boron enolates are formed under these
conditions and the products are 2,3-syn.
1) (Ipc) BOSO CF 3 CH
2
2
R (i-Pr) NEt 3 R′
CH 3 2 R
O 2) R′CH O
O OH
R′ = Me, n-Pr, i-Pr
CH 3 CH 3
R H R
H B R B
O Ipc O Ipc
O O
R CH
CH 3 3
Favored Disfavored
132
I. Paterson, J. M. Goodman, M. A. Lister, R. C. Schumann, C. K. McClure, and R. D. Norcross,
Tetrahedron, 46, 4663 (1990).