Page 143 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 143
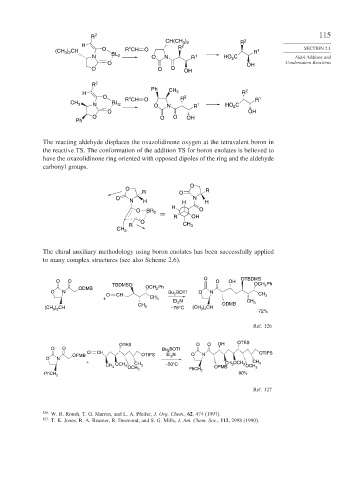
R 2 115
CH(CH 3 ) 2 R 2
H R 2
1
) CH O R CH O 1 SECTION 2.1
(CH 3 2 BL R
N 2 O N R 1 HO C Aldol Addition and
2
O OH Condensation Reactions
O O O OH
R 2
Ph 2
H CH 3 R
O 1 R 2 1
R CH O R
CH 3 N BL 2 O N R 1 HO C
2
O OH
O O O OH
Ph
The reacting aldehyde displaces the oxazolidinone oxygen at the tetravalent boron in
the reactive TS. The conformation of the addition TS for boron enolates is believed to
have the oxazolidinone ring oriented with opposed dipoles of the ring and the aldehyde
carbonyl groups.
O
O R
R O
O N
N H H H
H
O BR 2 O
R OH
O
R CH 3
CH 3
The chiral auxiliary methodology using boron enolates has been successfully applied
to many complex structures (see also Scheme 2.6).
O OTBDMS
O O O OH
TBDMSO OCH Ph
2
ODMB OCH Ph
2
O N Bu BOTf O N
O CH 2 CH 3
+ CH 3
Et N CH 3
CH 3 ODMB
) CH 3 –78°C (CH ) CH
(CH 3 2 3 2
72%
Ref. 126
OTES O O OH OTES
O O Bu 2 BOTf
O CH OTIPS
OPMB OTIPS Et N O N
3
O N
+ CH OCH CH
CH 3 OCH 3 CH 3 –50°C OPMB 3 3 OCH 3
OCH
3 PhCH 3
2
90%
PhCH 2
Ref. 127
126 W. R. Roush, T. G. Marron, and L. A. Pfeifer, J. Org. Chem., 62, 474 (1997).
127
T. K. Jones, R. A. Reamer, R. Desmond, and S. G. Mills, J. Am. Chem. Soc., 112, 2998 (1990).