Page 160 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 160
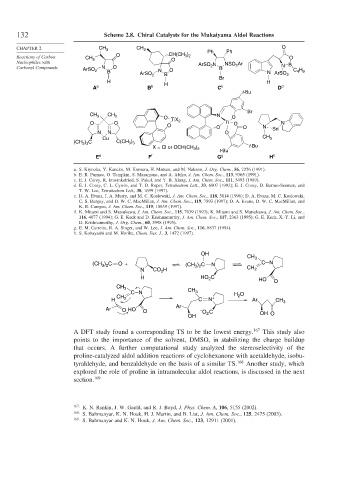
132 Scheme 2.8. Chiral Catalysts for the Mukaiyama Aldol Reactions
CHAPTER 2 CH 3 CH 3 O
Ph Ph
O CH(CH 3 ) 2
Reactions of Carbon CH 3 O O
Nucleophiles with ArSO 2 N NSO 2 Ar
Carbonyl Compounds N O B B N B
ArSO 2 N O C 4 H 9
B N
ArSO 2 B ArSO 2
Br
H H H
A a B b C c D d
t-Bu
Br
CH 3 CH 3 N
O O
TiX 2
O O Ti O N
O O O N
N N O Sn
Cu CH 3
(CH 3 ) 3 C C(CH 3 ) 3
t-Bu
X = Cl or OCH(CH 3 ) 2
t-Bu
E e F f G g H h
a. S. Kiyooka, Y. Kaneko, M. Komura, H. Matsuo, and M. Nakano, J. Org. Chem., 56, 2276 (1991).
b. E. R. Parmee, O. Tempkin, S. Masamune, and A. Abiko, J. Am. Chem. Soc., 113, 9365 (1991).
c. E. J. Corey, R. Imwinkelried, S. Pakul, and Y. B. Xiang, J. Am. Chem. Soc., 111, 5493 (1989).
d. E. J. Corey, C. L. Cywin, and T. D. Roper, Tetrahedron Lett., 33, 6907 (1992); E. J. Corey, D. Barnes-Seeman, and
T. W. Lee, Tetrahedron Lett., 38, 1699 (1997).
e. D. A. Evans, J. A. Murry, and M. C. Koslowski, J. Am. Chem. Soc., 118, 5814 (1996); D. A. Evans, M. C. Koslowski,
C. S. Burgey, and D. W. C. MacMillan, J. Am. Chem. Soc., 119, 7893 (1997); D. A. Evans, D. W. C. MacMillan, and
K. R. Campos, J. Am. Chem. Soc., 119, 10859 (1997).
f. K. Mitami and S. Matsukawa, J. Am. Chem. Soc., 115, 7039 (1993); K. Mitami and S. Matsukawa, J. Am. Chem. Soc.,
116, 4077 (1994); G. E. Keck and D. Krishnamurthy, J. Am. Chem. Soc., 117, 2363 (1995); G. E. Keck, X.-Y. Li, and
D. Krishnamurthy, J. Org. Chem., 60, 5998 (1995).
g. E. M. Carreira, R. A. Singer, and W. Lee, J. Am. Chem. Soc., 116, 8837 (1994).
h. S. Kobayashi and M. Horibe, Chem. Eur. J., 3, 1472 (1997).
OH
CH 3
(CH ) C O + (CH ) C N C N
3 2
3 2
N CO 2 H CH 2
H HO 2 C HO O
CH 3
C N CH 3 H O
CH + 2
H 2 C N Ar CH 3
Ar
Ar O HO O – O C
OH 2 OH O
A DFT study found a corresponding TS to be the lowest energy. 167 This study also
points to the importance of the solvent, DMSO, in stabilizing the charge buildup
that occurs. A further computational study analyzed the stereoselectivity of the
proline-catalyzed aldol addition reactions of cyclohexanone with acetaldehyde, isobu-
tyraldehyde, and benzaldehyde on the basis of a similar TS. 168 Another study, which
explored the role of proline in intramolecular aldol reactions, is discussed in the next
section. 169
167 K. N. Rankin, J. W. Gauld, and R. J. Boyd, J. Phys. Chem. A, 106, 5155 (2002).
168 S. Bahmanyar, K. N. Houk, H. J. Martin, and B. List, J. Am. Chem. Soc., 125, 2475 (2003).
169
S. Bahmanyar and K. N. Houk, J. Am. Chem. Soc., 123, 12911 (2001).