Page 772 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 772
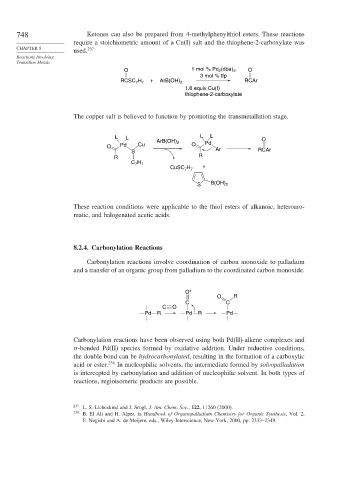
748 Ketones can also be prepared from 4-methylphenylthiol esters. These reactions
require a stoichiometric amount of a Cu(I) salt and the thiophene-2-carboxylate was
CHAPTER 8 237
used.
Reactions Involving
Transition Metals
O 1 mol % Pd 2 (dba) 3 O
3 mol % tfp
RCSC H + ArB(OH) 2 RCAr
7 7
1.6 equiv Cu(I)
thiophene-2-carboxylate
The copper salt is believed to function by promoting the transmetallation stage.
L L L L O
ArB(OH) 2 Pd
O Pd Cu O
S Ar RCAr
R R
C H
7 7
CuSC H +
7 7
S B(OH) 2
These reaction conditions were applicable to the thiol esters of alkanoic, heteroaro-
matic, and halogenated acetic acids.
8.2.4. Carbonylation Reactions
Carbonylation reactions involve coordination of carbon monoxide to palladium
and a transfer of an organic group from palladium to the coordinated carbon monoxide.
O +
O R
C C
C O
Pd R Pd R Pd
Carbonylation reactions have been observed using both Pd(II)-alkene complexes and
-bonded Pd(II) species formed by oxidative addition. Under reductive conditions,
the double bond can be hydrocarbonylated, resulting in the formation of a carboxylic
acid or ester. 238 In nucleophilic solvents, the intermediate formed by solvopalladation
is intercepted by carbonylation and addition of nucleophilic solvent. In both types of
reactions, regioisomeric products are possible.
237 L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 122, 11260 (2000).
238
B. El Ali and H. Alper, in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 2,
E. Negishi and A. de Meijere, eds., Wiley-Interscience, New York, 2000, pp. 2333–2349.