Page 776 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 776
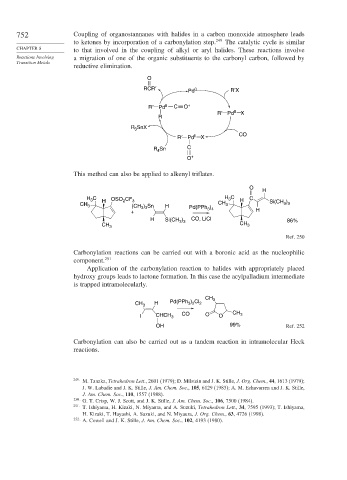
752 Coupling of organostannanes with halides in a carbon monoxide atmosphere leads
to ketones by incorporation of a carbonylation step. 249 The catalytic cycle is similar
CHAPTER 8 to that involved in the coupling of alkyl or aryl halides. These reactions involve
Reactions Involving a migration of one of the organic substituents to the carbonyl carbon, followed by
Transition Metals
reductive elimination.
O
RCR′ Pd 0 R′X
R′ Pd II C O +
R′ Pd II X
R
R 3 SnX
R′ Pd II X CO
R Sn C
4
O +
This method can also be applied to alkenyl triflates.
O
H
H C H OSO CF 3 H C H C
3
3
2
3 3
CH 3 (CH 3 3 H ) CH 3 Si(CH )
) Sn
+ Pd(PPh 3 4 H
H Si(CH ) CO, LiCl 86%
3 3
CH 3 CH 3
Ref. 250
Carbonylation reactions can be carried out with a boronic acid as the nucleophilic
component. 251
Application of the carbonylation reaction to halides with appropriately placed
hydroxy groups leads to lactone formation. In this case the acylpalladium intermediate
is trapped intramolecularly.
CH 3
) Cl
CH 3 H Pd(PPh 3 2 2
CO O CH 3
I CHCH 3 O
OH 99% Ref. 252
Carbonylation can also be carried out as a tandem reaction in intramolecular Heck
reactions.
249
M. Tanaka, Tetrahedron Lett., 2601 (1979); D. Milstein and J. K. Stille, J. Org. Chem., 44, 1613 (1979);
J. W. Labadie and J. K. Stille, J. Am. Chem. Soc., 105, 6129 (1983); A. M. Echavarren and J. K. Stille,
J. Am. Chem. Soc., 110, 1557 (1988).
250 G. T. Crisp, W. J. Scott, and J. K. Stille, J. Am. Chem. Soc., 106, 7500 (1984).
251 T. Ishiyama, H. Kizaki, N. Miyaura, and A. Suzuki, Tetrahedron Lett., 34, 7595 (1993); T. Ishiyama,
H. Kizaki, T. Hayashi, A. Suzuki, and N. Miyaura, J. Org. Chem., 63, 4726 (1998).
252
A. Cowell and J. K. Stille, J. Am. Chem. Soc., 102, 4193 (1980).