Page 774 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 774
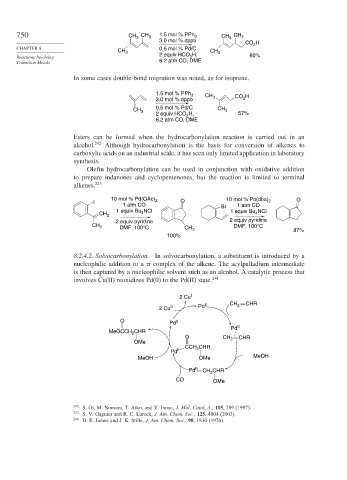
750 CH CH 3 1.5 mol % PPh 3 CH 3 CH 3
3
3.0 mol % dppb
CO H
2
CHAPTER 8 0.5 mol % Pd/C
CH 3 CH 3
2
Reactions Involving 2 equiv HCO H, 60%
Transition Metals 6.2 atm CO, DME
In some cases double-bond migration was noted, as for isoprene.
1.5 mol % PPh 3 CH CO H
3.0 mol % dppb 3 2
CH 3 0.5 mol % Pd/C CH 3
2 equiv HCO 2 H, 57%
6.2 atm CO, DME
Esters can be formed when the hydrocarbonylation reaction is carried out in an
alcohol. 242 Although hydrocarbonylation is the basis for conversion of alkenes to
carboxylic acids on an industrial scale, it has seen only limited application in laboratory
synthesis.
Olefin hydrocarbonylation can be used in conjunction with oxidative addition
to prepare indanones and cyclopentenones, but the reaction is limited to terminal
alkenes. 243
10 mol % Pd(OAc) 10 mol % Pd(dba) O
I 2 O 2
1 atm CO Br 1 atm CO
1 equiv Bu NCl 1 equiv Bu NCl
CH 2 4 4
2 equiv pyridine 2 equiv pyridine
CH 3 DMF, 100°C DMF, 100°C
CH 3
87%
100%
8.2.4.2. Solvocarbonylation. In solvocarbonylation, a substituent is introduced by a
nucleophilic addition to a complex of the alkene. The acylpalladium intermediate
is then captured by a nucleophilic solvent such as an alcohol. A catalytic process that
involves Cu(II) reoxidizes Pd(0) to the Pd(II) state. 244
2 Cu I
2 Cu II Pd II CH 2 CHR
O Pd 0
Pd II
MeOCCH 2 CHR
O CH 2 CHR
OMe
CCH CHR
2
Pd II
MeOH OMe MeOH
Pd II CH CHR
2
CO OMe
242
S. Oi, M. Nomura, T. Aiko, and Y. Inoue, J. Mol. Catal. A., 115, 289 (1997).
243 S. V. Gagnier and R. C. Larock, J. Am. Chem. Soc., 125, 4804 (2003).
244
D. E. James and J. K. Stille, J. Am. Chem. Soc., 98, 1810 (1976).