Page 779 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 779
Br 755
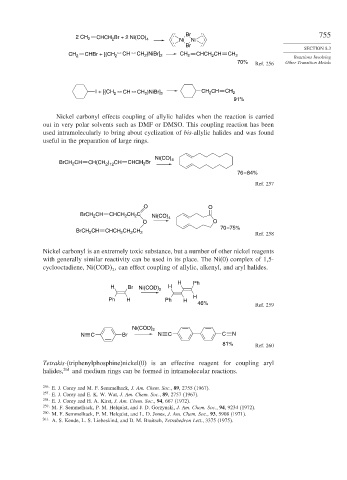
2 CH 2 CHCH Br + 2 Ni(CO) 4 Ni Ni
2
Br
SECTION 8.3
CH 2 CHBr + [(CH 2 CH CH )NiBr] 2 CH 2 CHCH CH CH 2 Reactions Involving
2
2
70% Ref. 256 Other Transition Metals
I + [(CH 2 CH CH )NiBr] 2 CH CH CH 2
2
2
91%
Nickel carbonyl effects coupling of allylic halides when the reaction is carried
out in very polar solvents such as DMF or DMSO. This coupling reaction has been
used intramolecularly to bring about cyclization of bis-allylic halides and was found
useful in the preparation of large rings.
Ni(CO) 4
BrCH CH CH(CH ) CH CHCH 2 Br
2
2 12
76 – 84%
Ref. 257
O O
BrCH CH CHCH 2 CH C Ni(CO) 4
2
2
O O
70 –75%
BrCH CH CHCH 2 CH CH 2 Ref. 258
2
2
Nickel carbonyl is an extremely toxic substance, but a number of other nickel reagents
with generally similar reactivity can be used in its place. The Ni(0) complex of 1,5-
cyclooctadiene, Ni COD , can effect coupling of allylic, alkenyl, and aryl halides.
2
H Ph
H Br Ni(COD) 2 H
H
Ph H Ph H
46%
Ref. 259
Ni(COD) 2
N C Br N C C N
81% Ref. 260
Tetrakis-(triphenylphosphine)nickel(0) is an effective reagent for coupling aryl
halides, 261 and medium rings can be formed in intramolecular reactions.
256 E. J. Corey and M. F. Semmelhack, J. Am. Chem. Soc., 89, 2755 (1967).
257
E. J. Corey and E. K. W. Wat, J. Am. Chem. Soc., 89, 2757 (1967).
258
E. J. Corey and H. A. Kirst, J. Am. Chem. Soc., 94, 667 (1972).
259 M. F. Semmelhack, P. M. Helquist, and J. D. Gorzynski, J. Am. Chem. Soc., 94, 9234 (1972).
260 M. F. Semmelhack, P. M. Helquist, and L. D. Jones, J. Am. Chem. Soc., 93, 5908 (1971).
261
A. S. Kende, L. S. Liebeskind, and D. M. Braitsch, Tetrahedron Lett., 3375 (1975).