Page 781 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 781
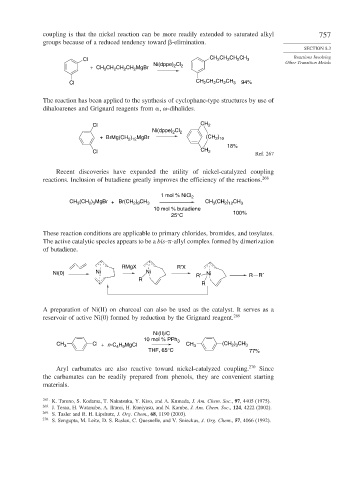
coupling is that the nickel reaction can be more readily extended to saturated alkyl 757
groups because of a reduced tendency toward -elimination.
SECTION 8.3
Reactions Involving
Cl CH 2 CH 2 CH 2 CH 3
Cl Other Transition Metals
Ni(dppe) 2
+ CH 3 CH 2 CH 2 CH 2 MgBr 2
Cl CH CH CH CH 3 94%
2
2
2
The reaction has been applied to the synthesis of cyclophane-type structures by use of
dihaloarenes and Grignard reagents from -dihalides.
Cl CH 2
Ni(dppe) Cl 2
2
)
+ BrMg(CH ) MgBr (CH 2 10
2 12
18%
Cl CH 2
Ref. 267
Recent discoveries have expanded the utility of nickel-catalyzed coupling
reactions. Inclusion of butadiene greatly improves the efficiency of the reactions. 268
1 mol % NiCl 2
) CH
CH (CH ) MgBr + Br(CH 2 9 3 CH (CH ) CH 3
2 3
3
2 12
3
10 mol % butadiene
25°C 100%
These reaction conditions are applicable to primary chlorides, bromides, and tosylates.
The active catalytic species appears to be a bis- -allyl complex formed by dimerization
of butadiene.
RMgX R′X
Ni(0) Ni Ni R′ Ni R R′
R
R
A preparation of Ni(II) on charcoal can also be used as the catalyst. It serves as a
reservoir of active Ni(0) formed by reduction by the Grignard reagent. 269
Ni(II)/C
10 mol % PPh 3
CH 3 Cl + n -C H MgCl CH 3 (CH ) CH 3
2 3
4 9
THF, 65°C 77%
Aryl carbamates are also reactive toward nickel-catalyzed coupling. 270 Since
the carbamates can be readily prepared from phenols, they are convenient starting
materials.
267
K. Tamno, S. Kodama, T. Nakatsuka, Y. Kiso, and A. Kumada, J. Am. Chem. Soc., 97, 4405 (1975).
268 J. Terao, H. Watanabe, A. Ikumi, H. Kuniyasu, and N. Kambe, J. Am. Chem. Soc., 124, 4222 (2002).
269 S. Tasler and R. H. Lipshutz, J. Org. Chem., 68, 1190 (2003).
270
S. Sengupta, M. Leite, D. S. Raslan, C. Quesnelle, and V. Snieckus, J. Org. Chem., 57, 4066 (1992).