Page 438 - Advanced thermodynamics for engineers
P. 438
428 CHAPTER 18 LIQUEFACTION OF GASES
a pressure high enough to enable the condensation to occur by heat transfer to a fluid at close to at-
mospheric temperature, depicted by point ‘h’ on Fig. 18.5(b); the pressure must also be below the
critical pressure for condensation to occur. The liquid that is produced at ‘j’ passes through the series of
throttles to obtain the refrigeration effect necessary to liquefy the carbon dioxide. In the last chamber,
the liquid is expanded below the triple point pressure, and the phase change enters the solid–gas region.
Carbon dioxide cannot exist as a liquid at atmospheric pressure, because its triple point pressure is
5.17 bar: hence it can either be a pressurised liquid or a solid. Dry ice is not in equilibrium with its
surroundings, and is continually evaporating. It could only be in a stable state, if the ambient tem-
perature was dropped to the equivalent of the saturated temperature for the solid state at a pressure of
1 bar.
18.2 LIQUEFACTION BY EXPANSION – METHOD (II)
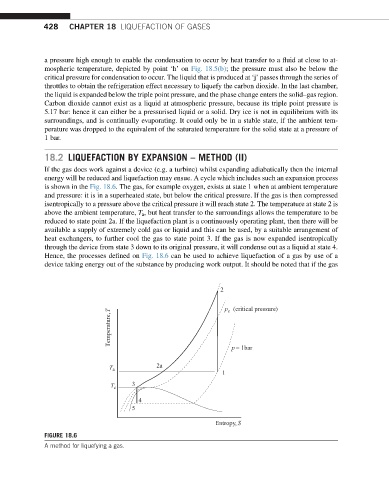
If the gas does work against a device (e.g. a turbine) whilst expanding adiabatically then the internal
energy will be reduced and liquefaction may ensue. A cycle which includes such an expansion process
is shown in the Fig. 18.6. The gas, for example oxygen, exists at state 1 when at ambient temperature
and pressure: it is in a superheated state, but below the critical pressure. If the gas is then compressed
isentropically to a pressure above the critical pressure it will reach state 2. The temperature at state 2 is
above the ambient temperature, T a , but heat transfer to the surroundings allows the temperature to be
reduced to state point 2a. If the liquefaction plant is a continuously operating plant, then there will be
available a supply of extremely cold gas or liquid and this can be used, by a suitable arrangement of
heat exchangers, to further cool the gas to state point 3. If the gas is now expanded isentropically
through the device from state 3 down to its original pressure, it will condense out as a liquid at state 4.
Hence, the processes defined on Fig. 18.6 can be used to achieve liquefaction of a gas by use of a
device taking energy out of the substance by producing work output. It should be noted that if the gas
2
Temperature, T p (critical pressure)
c
p = 1bar
T a 2a
1
3
T c
4
5
Entropy, S
FIGURE 18.6
A method for liquefying a gas.