Page 451 - Advanced thermodynamics for engineers
P. 451
18.2 LIQUEFACTION BY EXPANSION – METHOD (II) 441
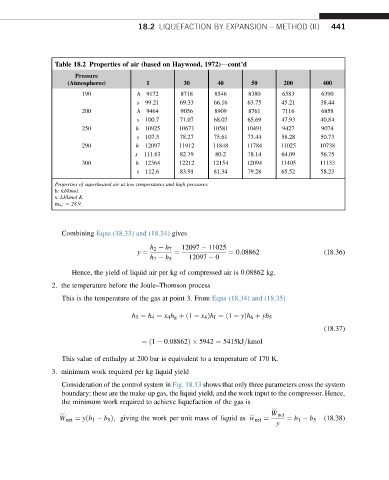
Table 18.2 Properties of air (based on Haywood, 1972)dcont’d
Pressure
(Atmospheres) 1 30 40 50 200 400
190 h 9172 8718 8546 8380 6583 6390
s 99.21 69.33 66.16 63.75 45.21 38.44
200 h 9464 9056 8909 8761 7116 6858
s 100.7 71.07 68.02 65.69 47.93 40.84
250 h 10925 10671 10581 10491 9427 9074
s 107.3 78.27 75.61 73.44 58.28 50.73
290 h 12097 11912 11848 11784 11025 10738
s 111.63 82.79 80.2 78.14 64.09 56.75
300 h 12364 12212 12154 12094 11405 11133
s 112.6 83.91 81.34 79.28 65.52 58.23
Properties of superheated air at low temperatures and high pressures.
h: kJ/kmol.
s: kJ/kmol K.
m w : ¼ 28.9.
Combining Eqns (18.33) and (18.34) gives
h 2 h 7 12097 11025
y ¼ ¼ ¼ 0:08862 (18.36)
h 7 h 5 12097 0
Hence, the yield of liquid air per kg of compressed air is 0.08862 kg.
2. the temperature before the Joule–Thomson process
This is the temperature of the gas at point 3. From Eqns (18.34) and (18.35)
h 3 ¼ h 4 ¼ x 4 h g þð1 x 4 Þh f ¼ð1 yÞh 6 þ yh 5
(18.37)
¼ð1 0:08862Þ 5942 ¼ 5415kJ=kmol
This value of enthalpy at 200 bar is equivalent to a temperature of 170 K.
3. minimum work required per kg liquid yield
Consideration of the control system in Fig. 18.13 shows that only three parameters cross the system
boundary; these are the make-up gas, the liquid yield, and the work input to the compressor. Hence,
the minimum work required to achieve liquefaction of the gas is
^
^ ^ W net
W net ¼ yðb 1 b 5 Þ; giving the work per unit mass of liquid as w net ¼ ¼ b 1 b 5 (18.38)
y