Page 450 - Advanced thermodynamics for engineers
P. 450
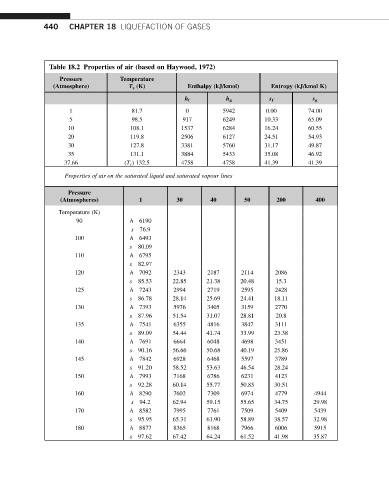
440 CHAPTER 18 LIQUEFACTION OF GASES
Table 18.2 Properties of air (based on Haywood, 1972)
Pressure Temperature
(Atmosphere) T s (K) Enthalpy (kJ/kmol) Entropy (kJ/kmol K)
h f h g s f s g
1 81.7 0 5942 0.00 74.00
5 98.5 917 6249 10.33 65.09
10 108.1 1537 6284 16.24 60.55
20 119.8 2506 6127 24.51 54.93
30 127.8 3381 5760 31.17 49.87
35 131.1 3884 5433 35.08 46.92
37.66 (T c ) 132.5 4758 4758 41.39 41.39
Properties of air on the saturated liquid and saturated vapour lines
Pressure
(Atmospheres) 1 30 40 50 200 400
Temperature (K)
90 h 6190
s 76.9
100 h 6493
s 80.09
110 h 6795
s 82.97
120 h 7092 2343 2187 2114 2086
s 85.53 22.85 21.38 20.48 15.3
125 h 7243 2994 2719 2595 2428
s 86.78 28.14 25.69 24.41 18.11
130 h 7393 5976 3405 3159 2770
s 87.96 51.54 31.07 28.81 20.8
135 h 7541 6355 4816 3847 3111
s 89.09 54.44 41.74 33.99 23.38
140 h 7691 6664 6048 4698 3451
s 90.16 56.66 50.68 40.19 25.86
145 h 7842 6928 6468 5597 3789
s 91.20 58.52 53.63 46.54 28.24
150 h 7993 7168 6786 6231 4123
s 92.28 60.14 55.77 50.85 30.51
160 h 8290 7602 7309 6974 4779 4944
s 94.2 62.94 59.15 55.65 34.75 29.98
170 h 8582 7995 7761 7509 5409 5439
s 95.95 65.31 61.90 58.89 38.57 32.98
180 h 8877 8365 8168 7966 6006 5915
s 97.62 67.42 64.24 61.52 41.98 35.87