Page 107 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 107
1522_C02.fm Page 92 Wednesday, November 12, 2003 9:36 AM
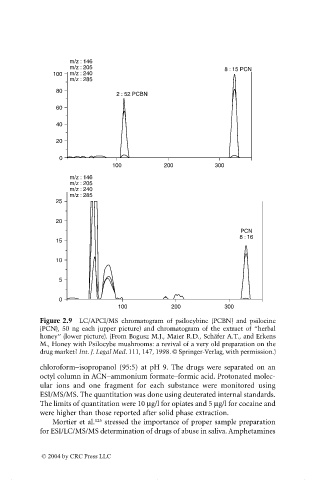
m/z : 146
m/z : 205 8 : 15 PCN
100 m/z : 240
m/z : 285
80
2 : 52 PCBN
60
40
20
0
100 200 300
m/z : 146
m/z : 205
m/z : 240
m/z : 285
25
20
PCN
8 : 16
15
10
5
0
100 200 300
Figure 2.9 LC/APCI/MS chromatogram of psilocybine (PCBN) and psilocine
(PCN), 50 ng each (upper picture) and chromatogram of the extract of “herbal
honey” (lower picture). (From Bogusz M.J., Maier R.D., Schäfer A.T., and Erkens
M., Honey with Psilocybe mushrooms: a revival of a very old preparation on the
drug market? Int. J. Legal Med. 111, 147, 1998. © Springer-Verlag, with permission.)
chloroform–isopropanol (95:5) at pH 9. The drugs were separated on an
octyl column in ACN–ammonium formate–formic acid. Protonated molec-
ular ions and one fragment for each substance were monitored using
ESI/MS/MS. The quantitation was done using deuterated internal standards.
The limits of quantitation were 10 mg/l for opiates and 5 mg/l for cocaine and
were higher than those reported after solid phase extraction.
125
Mortier et al. stressed the importance of proper sample preparation
for ESI/LC/MS/MS determination of drugs of abuse in saliva. Amphetamines
© 2004 by CRC Press LLC