Page 107 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 107
92 CONTROLLED-POTENTIAL TECHNIQUES
3-6.4 Detection Modes
The simplest, and by far the most common, detection scheme is the measurement of
the current at a constant potential. Such ®xed-potential amperometric measurements
have the advantage of being free of double-layer charging and surface-transient
effects. As a result, extremely low detection limitsÐon the order of 1±100 pg (about
10 14 moles of analyte)Ðcan be achieved. In various situations, however, it may be
desirable to change the potential during the detection (scan, pulse, etc.).
Potential-scanning detectors can increase the information content over that of
®xed-potential operation. By rapidly recording numerous voltammograms during the
elution, one obtains a three-dimensional detector response of the current against
potential and time. Such addition of the redox potential selectivity can offer
immediate identi®cation of eluting peaks, and helps in resolving chromatographi-
cally coeluting components. Different approaches to swept-potential detectors based
on square-wave voltammetry (13,14) or phase-sensitive AC voltammetry (63) have
been reported. The greater selectivity of potential-scanning detection is accompanied
by higher detection limits (versus ®xed-potential amperometry), because of the
additional background current associated with the potential scan.
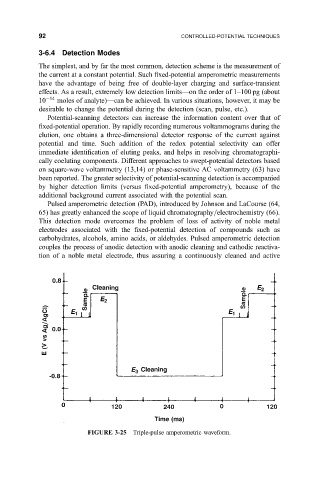
Pulsed amperometric detection (PAD), introduced by Johnson and LaCourse (64,
65) has greatly enhanced the scope of liquid chromatography=electrochemistry (66).
This detection mode overcomes the problem of loss of activity of noble metal
electrodes associated with the ®xed-potential detection of compounds such as
carbohydrates, alcohols, amino acids, or aldehydes. Pulsed amperometric detection
couples the process of anodic detection with anodic cleaning and cathodic reactiva-
tion of a noble metal electrode, thus assuring a continuously cleaned and active
FIGURE 3-25 Triple-pulse amperometric waveform.