Page 47 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 47
32 STUDY OF ELECTRODE REACTIONS
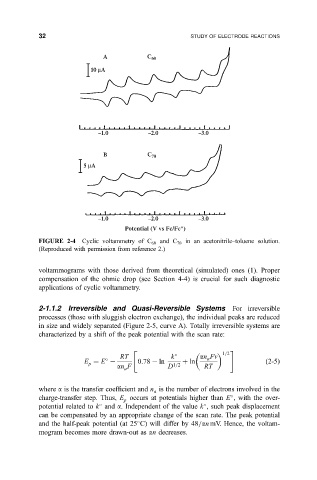
FIGURE 2-4 Cyclic voltammetry of C 60 and C 70 in an acetonitrile±toluene solution.
(Reproduced with permission from reference 2.)
voltammograms with those derived from theoretical (simulated) ones (1). Proper
compensation of the ohmic drop (see Section 4-4) is crucial for such diagnostic
applications of cyclic voltammetry.
2-1.1.2 Irreversible and Quasi-Reversible Systems For irreversible
processes (those with sluggish electron exchange), the individual peaks are reduced
in size and widely separated (Figure 2-5, curve A). Totally irreversible systems are
characterized by a shift of the peak potential with the scan rate:
" 1=2 #
RT k an Fv
a
E E 0:78 ln ln
2-5
p 1=2
an F D RT
a
where a is the transfer coef®cient and n is the number of electrons involved in the
a
charge-transfer step. Thus, E occurs at potentials higher than E , with the over-
p
potential related to k and a. Independent of the value k , such peak displacement
can be compensated by an appropriate change of the scan rate. The peak potential
and the half-peak potential (at 25 C) will differ by 48=an mV. Hence, the voltam-
mogram becomes more drawn-out as an decreases.