Page 49 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 49
34 STUDY OF ELECTRODE REACTIONS
For example, when the redox system is perturbed by a following chemical
reaction, that is, an EC mechanism,
O ne R ! Z
2-7
the cyclic voltammogram will exhibit a smaller reverse peak (because the product R
is chemically removed from the surface). The peak ratio i =i will thus be smaller
p;r p;f
than unity; the exact value of the peak ratio can be used to estimated the rate constant
of the chemical step. In the extreme case, the chemical reaction may be so fast that
all of R will be converted to Z, and no reverse peak will be observed. A classic
example of such an EC mechanism is the oxidation of the drug chlorpromazine to
form a radical cation that reacts with water to give an electroinactive sulfoxide.
Ligand exchange reactions (e.g., of iron porphyrin complexes) occurring after
electron transfer represent another example of such a mechanism.
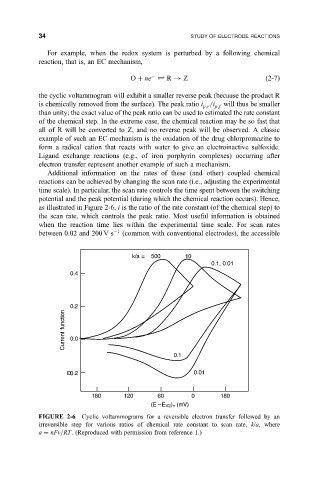
Additional information on the rates of these (and other) coupled chemical
reactions can be achieved by changing the scan rate (i.e., adjusting the experimental
time scale). In particular, the scan rate controls the time spent between the switching
potential and the peak potential (during which the chemical reaction occurs). Hence,
as illustrated in Figure 2-6, i is the ratio of the rate constant (of the chemical step) to
the scan rate, which controls the peak ratio. Most useful information is obtained
when the reaction time lies within the experimental time scale. For scan rates
between 0.02 and 200 V s 1 (common with conventional electrodes), the accessible
k/a = 500 10
0.1, 0.01
0.4
0.2
function
Current 0.0
0.1
Ð0.2 0.01
180 120 60 0 180
–
(E E V2) n (mV)
FIGURE 2-6 Cyclic voltammograms for a reversible electron transfer followed by an
irreversible step for various ratios of chemical rate constant to scan rate, k/a, where
a nFv=RT. (Reproduced with permission from reference 1.)