Page 53 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 53
38 STUDY OF ELECTRODE REACTIONS
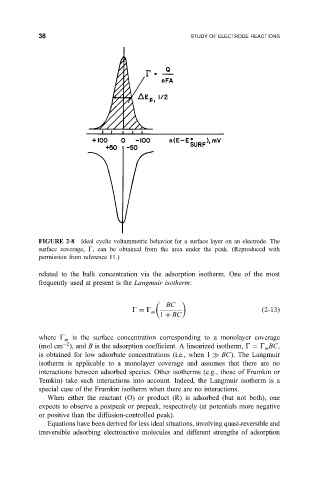
FIGURE 2-8 Ideal cyclic voltammetric behavior for a surface layer on an electrode. The
surface coverage, G, can be obtained from the area under the peak. (Reproduced with
permission from reference 11.)
related to the bulk concentration via the adsorption isotherm. One of the most
frequently used at present is the Langmuir isotherm:
BC
G G m
2-13
1 BC
where G is the surface concentration corresponding to a monolayer coverage
m
2
(mol cm ), and B is the adsorption coef®cient. A linearized isotherm, G G BC,
m
is obtained for low adsorbate concentrations (i.e., when 1 BC). The Langmuir
isotherm is applicable to a monolayer coverage and assumes that there are no
interactions between adsorbed species. Other isotherms (e.g., those of Frumkin or
Temkin) take such interactions into account. Indeed, the Langmuir isotherm is a
special case of the Frumkin isotherm when there are no interactions.
When either the reactant (O) or product (R) is adsorbed (but not both), one
expects to observe a postpeak or prepeak, respectively (at potentials more negative
or positive than the diffusion-controlled peak).
Equations have been derived for less ideal situations, involving quasi-reversible and
irreversible adsorbing electroactive molecules and different strengths of adsorption