Page 55 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 55
40 STUDY OF ELECTRODE REACTIONS
®rst one (in accordance with t 1=2 ). Background reactions, primarily those asso-
ciated with the double-layer charging and redox-surface processes, limit the detec-
tion limit to around the 1 10 5 M level. Background-subtracted cyclic
voltammetry can be employed for measuring lower concentrations (16). In particular,
1
fast-scan (1000 V s ) background-subtracted cyclic voltammetry is seeing
increased use for the in-vivo monitoring of neurotransmitters (such as dopamine
or serotonin) in the brain. Such coupling of digital background subtraction and fast
voltammetric measurements provides the subsecond temporal resolution necessary
for detecting dynamic concentration changes in the micromolar range occurring in
the extracellular environment of the brain. The good temporal and chemical
resolution of such in-vivo cyclic voltammetric experiments offers improved under-
standing of the chemistry of the brain. These repetitive scanning in-vivo experiments
generate large quantities of data that are best represented as three-dimensional
(potential, current, time) color contour images (17). For example, the temporal
release of dopamine following electrical stimulation is evidenced from the rapid
increase in color around its peak potential. The ultrafast scanning also eliminates
interferences from adsorption processes and chemical reactions that are coupled to
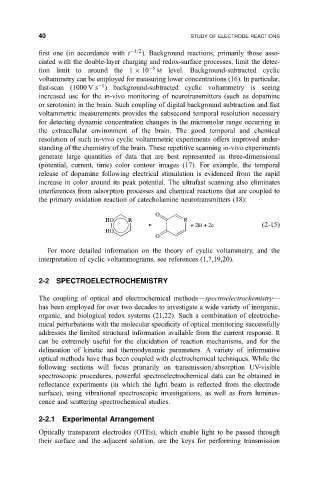
the primary oxidation reaction of catecholamine neurotransmitters (18):
O
HO R R
+ 2H + 2e
2-15
HO
O
For more detailed information on the theory of cyclic voltammetry, and the
interpretation of cyclic voltammograms, see references (1,7,19,20).
2-2 SPECTROELECTROCHEMISTRY
The coupling of optical and electrochemical methodsÐspectroelectrochemistryÐ
has been employed for over two decades to investigate a wide variety of inorganic,
organic, and biological redox systems (21,22). Such a combination of electroche-
mical perturbations with the molecular speci®city of optical monitoring successfully
addresses the limited structural information available from the current response. It
can be extremely useful for the elucidation of reaction mechanisms, and for the
delineation of kinetic and thermodynamic parameters. A variety of informative
optical methods have thus been coupled with electrochemical techniques. While the
following sections will focus primarily on transmission=absorption UV-visible
spectroscopic procedures, powerful spectroelectrochemical data can be obtained in
re¯ectance experiments (in which the light beam is re¯ected from the electrode
surface), using vibrational spectroscopic investigations, as well as from lumines-
cence and scattering spectrochemical studies.
2-2.1 Experimental Arrangement
Optically transparent electrodes (OTEs), which enable light to be passed through
their surface and the adjacent solution, are the keys for performing transmission