Page 360 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 360
Packed Towers 349
y* = 1.5~ Note that the graphical integration is never exact and
y" = 1.5 (.1048) = 0.157 hence the correction often makes little difference except
Calculate (1 - y): for cases of curved equilibrium lines.
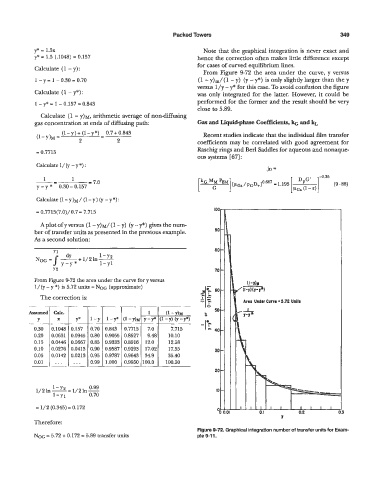
From Figure 9-72 the area under the curve, y versus
1
1 - y = 1 - 0.30 9 0.70 (1 - Y)~/( - y) (y - y*) is only slightly larger than the y
versus l/y - y* for this case. To avoid confusion the figure
Calculate (1 - y*) : was only integrated for the latter. However, it could be
1 - y* = 1 - 0.157 - 0.843 performed for the former and the result should be very
close to 5.89.
Calculate (1 - Y)M, arithmetic average of non-dihing
gas concentration at ends of diffusing path: Gap and Liquid-phase Coefficients, and kL
- -
(1 - y) + (1 - y*) 0.7 + 0.843 Recent studies indicate that the individual film transfer
(1-Y)h.l-
2 2 coefficients may be correlated with good agreement for
= 0.7715 Raschig rings and Berl Saddles for aqueous and nonaque-
ous systems [67]:
Calculate 1/ (y - y *) :
jD =
1 1
-IC = 7.0
y - y * 0.30 - 0.157
(1
Calculate (1 - y)~/ - y) (y - y*):
= 0.7715(7.0)/0.7= 7.715 ""inl
A plot of y versus (1 - Y)M/ (1 - y) (y - y*) gives the num-
ber of transfer units as presented in the previous example. 90
As a second solution:
il
N0G -J -5+1/2ln- l-yn
.. 1-yl
Y2 70
From Figure 9-72 the area under the curve for y versus
l/(y - y *) is 5.72 units = NOG (approximate)
The correction is: Art0 Under Curvr ~5.72 Units
... - -_ -
1,
Assumed CalC. (1 -Y)M Y-Y*
Y*
___ Y - X . ._
0.30 0.1048 0.157
0.20 0.0631 0.0945
0.15 0.0446 0.0667
0.10 0.0276 0.0413
0.05 0.0142 0.0213
0.01 ... ...
I-VZ - 0 99
1/2
1/2 In - In-
I-Yl 0.70
= 1/2 (0.345) = 0.172
I
Y
Therefore:
Figure 9-72. Graphical integration number of transfer units for Exam-
NOG = 5.72 + 0.172 = 5.89 transfer units ple 9-1 1.