Page 363 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 363
352 Applied Process Design for Chemical and Petrochemical Plants
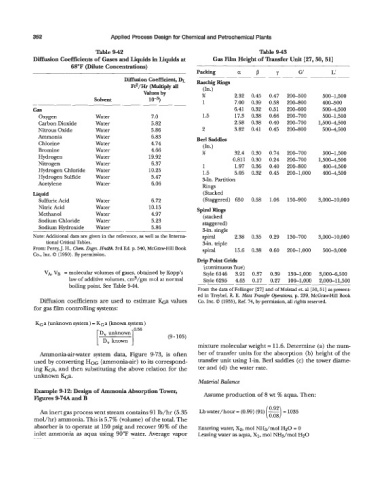
Table 9-42 Table 9-43
Diffusion Coefficients of Gases and Liquids in Liquids at Gas Film Height of Transfer Unit [27,50,51]
68°F (Dilute Concentrations)
Packing a P Y G' L'
Diffusion Coefficient, DL RaschigRhlgs ~ ~
~
Ftz/Hr (Multiply all (In.)
Values by 36 2.32 0.45 0.47
Solvent 10-5) 1 7.00 0.39 0.58 200-500 500-1,500
~ 200-800 400-500
Gas 6.41 0.32 0.51 200-600 5004,500
Oxygen Water 7.0 1.5 17.3 0.38 0.66 200-700 500-1,500
Carbon Dioxide Water 5.82 2.58 0.38 0.40 200-700 1,5004,500
Nitrous Oxide Water 5.86 2 3.82 0.41 0.45 200-800 5004,500
Ammonia Water 6.83 Berl Saddles
Chlorine Water 4.74
(In.)
Bromine Water 4.66 % 32.4 0.30 0.74 200-700 500-1,500
Hydrogen Water 19.92 0.811 0.30 0.24 200-700 1,500-4,500
Nitrogen Water 6.37 1 1.97 0.36 0.40 200-800 4004,500
Hydrogen Chloride Water 10.25 1.5 5.05 0.32 0.45 200-1,000 4004,500
Hydrogen Sulfide Water 5.47 3-In. Partition
Acetylene Water 6.06 Rings
Liquid (Stacked
Sulfuric Acid Water 6.72 (Staggered) 650 0.58 1.06 150-900 3,000-10,000
Nitric Acid Water 10.15 spiral Rings
Methanol Water 4.97 (stacked
Sodium Chloride Water 5.23 staggered)
Sodium Hydroxide Water 5.86 %in. single
Note: Additional data are given in the reference, as well as the Interna- spiral 2.38 0.35 0.29 130-700 3,000-10,000
tional Critical Tables. 3-in. triple
From: Perry, J. H., Cha. Engrs. Hndbk. 3rd Ed. p. 540, McGraw-Hill Book spiral 15.6 0.38 0.60 200-1,000 500-3,000
Co., Inc. 0 (1950). By permission.
Drip Point Grids
(continuous flue)
VA, VB = molecular volumes of gases, obtained by Kopp's Style 6146 3.91 0.37 0.39 130-1,000 3,0004,500
law of additive volumes, cm3/gm mol at normal Style 6295 4.65 0.17 0.27 100-1,000 2,000-11,500
boiling point. See Table 9-44.
From the data of Fellinger [27] Fd of Molstad et. al [30,51] as present-
ed in Treybel, R. E. Mass Transfer Opmutions, p. 239, McGraw-Hill Book
Diffusion coefficients are used to estimate &a values Co. Inc. 0 (1955), Ref. 74, by permission, all rights reserved.
for gas film controlling systems:
KGa (unknown system) = KGa (known system)
[ D, known ] 0.56 (9 - 105)
D, unknown
mixture molecular weight = 11.6. Determine (a) the num-
Ammonia-air-water system data, Figure 9-73, is often ber of transfer units for the absorption (b) height of the
used by converting HOG (ammonia-air) to its correspond- transfer unit using 1-in. Berl saddles (c) the tower diame-
ing &a, and then substituting the above relation for the ter and (d) the water rate.
unknown ka.
Material Balance
Example 9-12: Design of Ammonia Absorption Tower, Assume production of 8 wt % aqua. Then:
Figures 9-74A and B
( Z)
An inert gas process vent stream contains 91 lb/hr (5.35 Lb water/ hour = (0.99) (91) - = 1035
mol/hr) ammonia. This is 5.7% (volume) of the total. The
absorber is to operate at 150 psig and recover 99% of the Entering water, Xz, mol NHs/mol H20 = 0
inlet ammonia as aqua using 90°F water. Average vapor Leaving water as aqua, XI, mol NHg/mol H20