Page 364 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 364
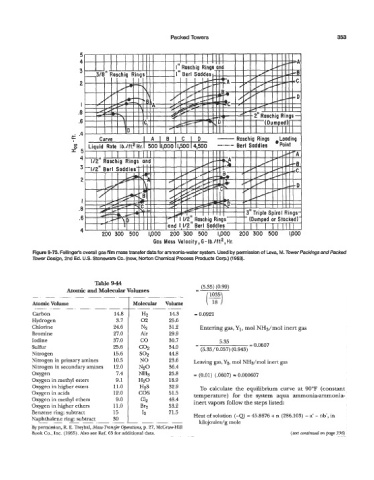
Packed Towers 353
Figure 9-73. Fellinger’s overall gas film mass transfer data for ammonia-water system. Used by permission of Leva, M. Tower facldngs and Packed
Tower Design, 2nd Ed. US. Stoneware Co. (now, Norton Chemical Process Products Corp.) (1 953).
- (5.35) (0.99)
-
-. . .- -. .-.. -. . .-
Atomic Volume Molecular Volume
_-
. -. . . . . .- . -, .- . __ . .-
Carbon 14.8 H2 14.3 = 0.0921
Hydrogen 3.7 02 25.6
Chlorine 24.6 N2 31.2 Entering gas, Y1, mol NHs/mol inert gas
Bromine 27.0 Air 29.9
Iodine 37.0 co 30.7 5.35
Sulfur 25.6 GO2 34.0 = (5.35/0.057) (0.943) = 0.0607
Nitrogen 15.6 SO2 44.8
Nitrogen in primary amines 10.5 NO 23.6 Leaving gas, Y2, mol NH3/mol inert gas
Nitrogen in secondary amines 12.0 N20 36.4
Oxygen 7.4 NH3 25.8 = (0.01) (.0607) = 0.000607
Oxygen in methyl esters 9.1 H20 18.9
Oxygen in higher esters 11.0 HZS 32.9 To calculate the equilibrium curve at 90°F (constant
Oxygen in acids 12.0 cos 51.5 temperature) for the system aqua ammonia-ammonia-
Oxygen in methyl ethers 9.0 C12 48.4 inert vapors follow the steps listed:
Oxygen in higher ethers 11.0 B‘2 53.2
Benzene ring: subtract 15 I2 71.5
Naphthalene ring: subtract 30 Heat of solution (-Q = 45.8676 + n (286.103) - a’ - nb‘, in
-.
- -_ .- - __ kilojoules/g mole
(text continued on page 35@