Page 38 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 38
Distillation 27
pB XB
Temp"C (PR-PT) (JG-P~T) XR . = B y z
80 480 480 1.0 0 1.0 0
90 590 350 0.593 0.407 0.780 0.220
100 770 210 0.273 0.727 0.474 0.526
110 1,000 20 0.0 0.980 0.0457 0.954 11w
111.5 1,000 0 0.0 1.0 0.0 1.0
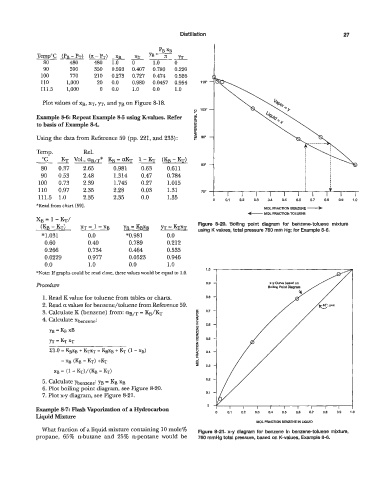
Plot values of xg, XT, YT, and yg on Figure 8-18.
p 100'
Example 86: Repeat Example 8-5 using K-values. Refer ui
K
to basis of Example 8-4. z
a
Using the data from Reference 59 (pp. 221, and 233): 90'
Temp. Re1 .
KT Vol., ag/~* Kg =UKT 1 - KT (Kg - KT)
--
"C
80 0.37 2.65 0.981 0.63 0.611
90 0.33 2.48 1.314 0.47 0.784
100 0.73 2.39 1.745 0.27 1.015
110 0.97 2.35 2.28 0.03 1.31 70'
111.5 1.0 2.35 2.35 0.0 1.35 0 0.1 0.2 0.3 OA 0.5 0.6 0.7 0.8 0.9 1.0
*Read from chart [59].
MOL FRACTION BENZENE
MOL FRACTION TOLUENE
XB = 1 - KT/
(KB - KT) XT 1 - Xg YB = KBXB YT = KTXT Figure 8-20. Boiling point diagram for benzene-toluene mixture
using K values, total pressure 760 mm Hg; for Example 8-6.
*1.031 0.0 80.981 0.0
0.60 0.40 0.789 0.212
0.266 0.734 0.464 0.535
0.0229 0.977 0.0523 0.946
0.0 1 .o 0.0 1 .o
1 .o
*Note: If graphs could be read close, these values would be equal to 1 .O.
based
Cuwe
x-y
x-y Cuwe based on
on
Procedure 0.9 Boillng Point Diagram
Diagram
Boillng
Point
1. Read K value for toluene from tables or charts. 0.8
2. Read CY. values for benzene/toluene from Reference 59.
3. Calculate K (benzene) from: ag/~ KB/KT 6 0.7
=
4. Calculate Xbenzene: 3
f 0.6
YB'GXB fi
0.5
YT = KT xT 5
Z1.0 = KBXB + KTXT = KBXB + KT (1 - XB) $ 0.4
U
XB (% - KT) +KT d
0.3
XB = (1 - KT)/(KB - KT)
0.2
5. calculate Ybemene: yB = XB
6. Plot boiling point diagram, see Figure 8-20.
7. Plot x-y diagram, see Figure 8-21. 0.1
0 V
1 1
1 1
1 1
1 1
1 1
1 1
1 1
Example &?: Flash Vaporization of a Hydrocarbon 0 0.1 1 1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 IO
Liquid Mixture
MOL FRACTION BENZENE IN LIQUID
What fraction of a liquid mixture containing 10 mole% Figure 8-21. x-y diagram for benzene in benzene-toluene mixture,
propane, 65% n-butane and 25% n-pentane would be 760 rnrnHg total pressure, based on K-values, Example 8-6.