Page 42 - Artificial Intelligence for Computational Modeling of the Heart
P. 42
12 Chapter 1 Multi-scale models of the heart for patient-specific simulations
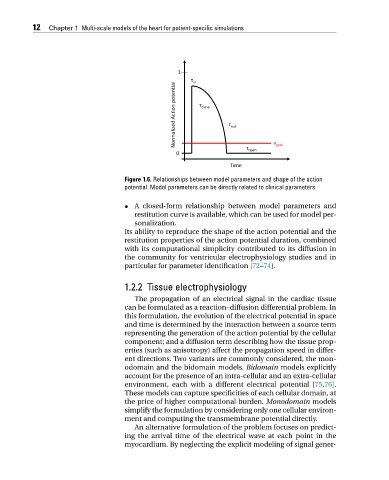
Figure 1.6. Relationships between model parameters and shape of the action
potential. Model parameters can be directly related to clinical parameters.
• A closed-form relationship between model parameters and
restitution curve is available, which can be used for model per-
sonalization.
Its ability to reproduce the shape of the action potential and the
restitution properties of the action potential duration, combined
with its computational simplicity contributed to its diffusion in
the community for ventricular electrophysiology studies and in
particular for parameter identification [72–74].
1.2.2 Tissue electrophysiology
The propagation of an electrical signal in the cardiac tissue
can be formulated as a reaction–diffusion differential problem. In
this formulation, the evolution of the electrical potential in space
and time is determined by the interaction between a source term
representing the generation of the action potential by the cellular
component; and a diffusion term describing how the tissue prop-
erties (such as anisotropy) affect the propagation speed in differ-
ent directions. Two variants are commonly considered, the mon-
odomain and the bidomain models. Bidomain models explicitly
account for the presence of an intra-cellular and an extra-cellular
environment, each with a different electrical potential [75,76].
These models can capture specificities of each cellular domain, at
the price of higher computational burden. Monodomain models
simplify the formulation by considering only one cellular environ-
ment and computing the transmembrane potential directly.
An alternative formulation of the problem focuses on predict-
ing the arrival time of the electrical wave at each point in the
myocardium. By neglecting the explicit modeling of signal gener-