Page 37 - Artificial Intelligence for Computational Modeling of the Heart
P. 37
Chapter 1 Multi-scale models of the heart for patient-specific simulations 7
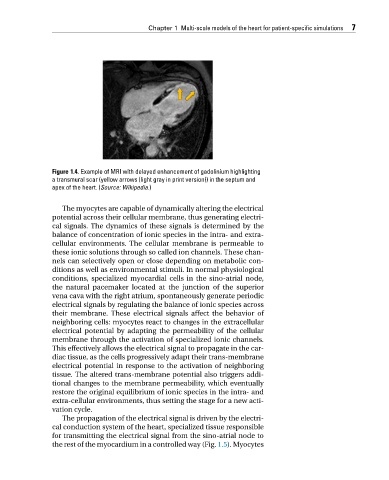
Figure 1.4. Example of MRI with delayed enhancement of gadolinium highlighting
a transmural scar (yellow arrows (light gray in print version)) in the septum and
apex of the heart. (Source: Wikipedia.)
The myocytes are capable of dynamically altering the electrical
potential across their cellular membrane, thus generating electri-
cal signals. The dynamics of these signals is determined by the
balance of concentration of ionic species in the intra- and extra-
cellular environments. The cellular membrane is permeable to
these ionic solutions through so called ion channels. These chan-
nels can selectively open or close depending on metabolic con-
ditions as well as environmental stimuli. In normal physiological
conditions, specialized myocardial cells in the sino-atrial node,
the natural pacemaker located at the junction of the superior
vena cava with the right atrium, spontaneously generate periodic
electrical signals by regulating the balance of ionic species across
their membrane. These electrical signals affect the behavior of
neighboring cells: myocytes react to changes in the extracellular
electrical potential by adapting the permeability of the cellular
membrane through the activation of specialized ionic channels.
This effectively allows the electrical signal to propagate in the car-
diac tissue, as the cells progressively adapt their trans-membrane
electrical potential in response to the activation of neighboring
tissue. The altered trans-membrane potential also triggers addi-
tional changes to the membrane permeability, which eventually
restore the original equilibrium of ionic species in the intra- and
extra-cellular environments, thus setting the stage for a new acti-
vation cycle.
The propagation of the electrical signal is driven by the electri-
cal conduction system of the heart, specialized tissue responsible
for transmitting the electrical signal from the sino-atrial node to
the rest of the myocardium in a controlled way (Fig. 1.5). Myocytes