Page 35 - Artificial Intelligence for Computational Modeling of the Heart
P. 35
Chapter 1 Multi-scale models of the heart for patient-specific simulations 5
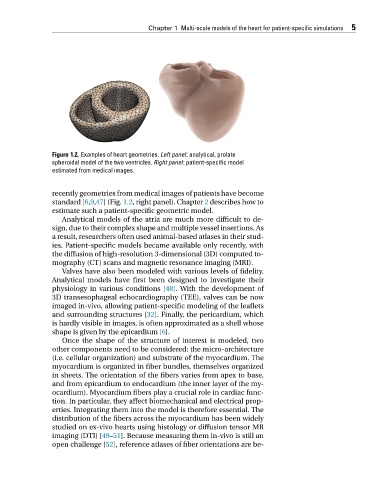
Figure 1.2. Examples of heart geometries. Left panel: analytical, prolate
spheroidal model of the two ventricles. Right panel: patient-specific model
estimated from medical images.
recently geometries from medical images of patients have become
standard [6,9,47](Fig. 1.2,rightpanel).Chapter 2 describes how to
estimate such a patient-specific geometric model.
Analytical models of the atria are much more difficult to de-
sign, due to their complex shape and multiple vessel insertions. As
a result, researchers often used animal-based atlases in their stud-
ies. Patient-specific models became available only recently, with
the diffusion of high-resolution 3-dimensional (3D) computed to-
mography (CT) scans and magnetic resonance imaging (MRI).
Valves have also been modeled with various levels of fidelity.
Analytical models have first been designed to investigate their
physiology in various conditions [48]. With the development of
3D transesophageal echocardiography (TEE), valves can be now
imaged in-vivo, allowing patient-specific modeling of the leaflets
and surrounding structures [32]. Finally, the pericardium, which
is hardly visible in images, is often approximated as a shell whose
shapeisgiven bytheepicardium[6].
Once the shape of the structure of interest is modeled, two
other components need to be considered: the micro-architecture
(i.e. cellular organization) and substrate of the myocardium. The
myocardium is organized in fiber bundles, themselves organized
in sheets. The orientation of the fibers varies from apex to base,
and from epicardium to endocardium (the inner layer of the my-
ocardium). Myocardium fibers play a crucial role in cardiac func-
tion. In particular, they affect biomechanical and electrical prop-
erties. Integrating them into the model is therefore essential. The
distribution of the fibers across the myocardium has been widely
studied on ex-vivo hearts using histology or diffusion tensor MR
imaging (DTI) [49–51]. Because measuring them in-vivo is still an
open challenge [52], reference atlases of fiber orientations are be-