Page 228 - Battery Reference Book
P. 228
18/12 Lead-acid secondary batteries
-
Hardening by alloying (natural hardness) N
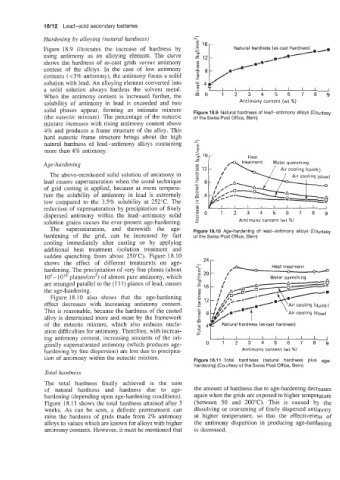
Figure 18.9 illustrates the increase of hardness by
using antimony as an alloying element. The curve
shows the hardness of as-cast grids versus antimony
content of the alloys. In the case of low antimony
contents (t3% antimony), the antimony forms a solid
solution with lead. An alloying element converted into 41/
c
a solid solution always hardens the solvent metal. so7 I 1 ; k ; A ;
When the antimony content is increased further, the
solubility of antimony in lead is exceeded and two Antimony content (wt %)
solid phases appear, forming an intimate mixture Figure 18.9 Natural hardness of lead-antimony alloys (Courtesy
(the eutectic mixture). The percentage of the eutectic of the Swiss Post Office, Bern)
mixture increases with rising antimony content above
4% and produces a frame structure of the alloy. This
hard eutectic frame structure brings about the high -
natural hardness of lead-antimony alloys containing N E
E
more than 4% antimony. .
b 16
Age-hurdening
12
The above-mentioned solid solution of antimony in
lead causes supersaturation when the usual technique
of grid casting is applied, because at room tempera-
ture the solubility of antimony in lead is extremely
low compared to the 3.5% solubility at 252°C. The ._
reduction of supersaturation by precipitation of finely
dispersed antimony within the lead-antimony solid Z 0 O 1 2 3 4 5 6 7 8 9
solution grains causes the ever-present age-hardening. - Antimony content (wt %)
The supersaturation, and therewith the age- Figure 18.10 Age-hardening of lead-antimony alloys (Courtesy
hardening of the grid, can be increased by fast of the Swiss Post Office, Bern)
cooling immediately after casting or by applying
additional heat treatment (solution treatment and
sudden quenching from about 250°C). Figure 18.10
shows the effect of different treatments on age- 24 ,-
hardening. The precipitation of very fine planes (about
105-1010 planes/cm2) of almost pure antimony, which
are arranged parallel to the (1 11) planes of lead, causes
the age-hardening.
Figure 18.10 also shows that the age-hardening
effect decreases with increasing antimony content.
This is reasonable, because the hardness of the casted
alloy is determined more and more by the framework
of the eutectic mixture, which also reduces nucle-
ation difficulties for antimony. Therefore, with increas-
ing antimony content, increasing amounts of the ori-
ginally supersaturated antimony (which produces age- 0 1 2 3 4 5 6 7 8 9
hardening by fine dispersion) are lost due to precipita- Antimony content (wt %I
tion of antimony within the eutectic mixture. Figure 18.11 Total hardness (natural hardness plus age-
hardening) (Courtesy of the Swiss Post Office, Bern)
Total hardness
The total hardness finally achieved is the sum
of natural hardness and hardness due to age- the amount of hardness due to age-hardening decreases
hardening (depending upon age-hardening conditions). again when the grids are exposed to higher temperature
Figure 18.11 shows the total hardness attained after 3 (between 50 and 200°C). This is caused by the
weeks. As can be seen, a definite pretreatment can dissolving or coarsening of finely dispersed antimony
raise the hardness of grids made from 2% antimony at higher temperature, so that the effectiveness of
alloys to values which are known for alloys with higher the antimony dispersion in producing age-hardening
antimony contents. However, it must be mentioned that is decreased.