Page 230 - Battery Reference Book
P. 230
18/14 Lead-acid secondary batteries
increase the castability) with lead, it is feasible to selenium alloys. This results in capacity stability as
manufacture battery grids with very low antimony con- well as good cycling performance.
tents, which achieve the necessary mechanical strength Because of the extremely high potential of the pos-
within suitable ageing times, and show the necessary itive electrode, only lead can be used as grid material in
grid quality. stationary and, indeed, all types of lead-acid battery.
This lead is unavoidably subject to gradual erosion
from corrosion. For battery applications this corrosion
18.4 Lead alloy development in rate must be reasonably low, otherwise the battery life
standby (stationary) batteries is limited by the corrosion rate of the positive grid.
The potential of the electrode (the most important
Until 10 years ago, the grids of lead-acid batteries parameter) determines the electrochemical corrosion
were usually made of lead-antimony alloys contain-
ing 5- 11 wt% antimony. The necessary mechanical of lead. Therefore, potentiostatic corrosion tests are
strength and castability are easily achieved with this very suited to comparing corrosion rates for different
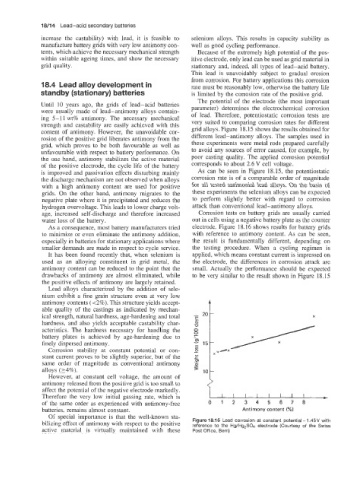
content of antimony. However, the unavoidable cor- grid alloys. Figure 18.15 shows the results obtained for
rosion of the positive grid liberates antimony from the different lead-antimony alloys. The samples used in
grid, which proves to be both favourable as well as these experiments were metal rods prepared carefully
unfavourable with respect to battery performance. On to avoid any sources of error caused, for example, by
the one hand, antimony stabilizes the active material poor casting quality. The applied corrosion potential
of the positive electrode, the cycle life of the battery corresponds to about 2.6 V cell voltage.
is improved and passivation effects disturbing mainly As can be seen in Figure 18.15, the potentiostatic
the discharge mechanism are not observed when alloys corrosion rate is of a comparable order of magnitude
with a high antimony content are used for positive for all tested antimonial lead alloys. On the basis of
grids. On the other hand, antimony migrates to the these experiments the selenium alloys can be expected
negative plate where it is precipitated and reduces the to perform slightly better with regard to corrosion
hydrogen overvoltage. This leads to lower charge volt- attack than conventional lead-antimony alloys.
age: increased self-discharge and therefore increased Corrosion tests on battery grids are usually carried
water loss of the battery. out in cells using a negative battery plate as the counter
As a consequence, most battery manufacturers tried electrode. Figure 18.16 shows results for battery grids
to minimize or even eliminate the antimony addition, with reference to antimony content. As can be seen,
especially in batteries for stationary applications where the result is fundamentally different, depending on
smaller demands are made in respect to cycle service. the testing procedure. When a cycling regimen is
It has been found recently that, when selenium is applied, which means constant current is impressed on
used as an alloying constituent in grid metal, the the electrode, the differences in corrosion attack are
antimony content can be reduced to the point that the small. Actually the performance should be expected
drawbacks of antimony are almost eliminated, while to be very similar to the result shown in Figure 18.15
the positive effects of antimony are largely retained.
Lead alloys characterized by the addition of sele-
nium exhibit a fine grain structure even at very low
antimony contents (12%). This structure yields accept-
able quality of the castings as indicated by mechan-
ical strength, natural hardness, age-hardening and total X
hardness, and also yields acceptable castability char-
acteristics. The hardness necessary for handling the
battery plates is achieved by age-hardening due to
finely dispersed antimony.
Corrosion stability at constant potential or con-
stant current proves to be slightly superior, but of the
same order of magnitude as conventional antimony
alloys (14%).
However, at constant cell voltage, the amount of
antimony released from the positive grid is too small to
affect the potential of the negative electrode markedly.
Therefore the very low initial gassing rate, which is
of the same order as experienced with antimony-free 0 1 2 3 4 5 6 7 8
batteries, remains almost constant. Antimony content (%)
Of special importance is that the well-known sta-
Figure 18.15 Lead corrosion at constant potential - 1.45V with
bilizing effect of antimony with respect to the positive reference to the Hg/Hg,S04 electrode (Courtesy of the Swiss
active material is virtually maintained with these Post Office, Bern)